Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Could Sulodexide be Helpful in COVID-19?
- Alberto C Frati Munari*;
- Internist, Internal Medicine, Hospital Medica Sur, Mexico.
- Sergio O Flores Cantu;
- Angiologist and vascular surgeon, Angiology Vascular Therapy. Hospital Christus Muguerza Alta Especialidad, Monterrey, Nuevo Leon. Mexico.
- Nora E Lecuona Huet;
- Angiologist and vascular surgeon, ConCIENCIA Vascular. Mexico City. Mexico.
- Miguel A Bautista Alfaro
- Alfasigma Laboratory, Mexico.

| Received | : | Dec 20, 2020 |
| Accepted | : | Feb 02, 2021 |
| Published Online | : | Feb 05, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Frati AC, Flores SO, Lecuona NE, Bautista MA. Could sulodexide be helpful in COVID-19?. Ann Cardiol Vasc Med. 2021: 4(1); 1040.
Abstract
Vascular disorder is prominent in COVID-19, mainly characterized by endothelial damage, severe inflammation and thrombotic trend. We reviewed the data suggesting that sulodexide, a glycosaminoglycan with endothelial protective, vascular anti-inflammatory, and antithromotic activities, could be helpful in this disease.
Introduction
The Coronavirus SARS Cov-2 is a highly contagious virus causing a pandemic with several millions of infected people in the whole world. The disease caused by this virus, called COVID-19, has wide clinical spectrum, ranging from asymptomatic, mild or severe symptoms and death, with a high fatality-rate, manly in the elderly [1]. The main clinical manifestations are respiratory, although extrapulmonary involvement as thrombotic complications, myocardial dysfunction, gastrointestinal symptoms, hepatocellular and kidney injury, as well as dermatologic rash are recognized [2].
Mortality of COVID-19 is still high despite all therapeutic measures. Few treatments have shown to improve severely ill patients in intensive care unit as corticosteroids, thrombo-prophylaxis with low-molecular-weight heparin, and remdesivir [1,3]. However, up to October 2020 no effective treatment has been approved, including therapy for ambulatory subjects not so severely ill, where the goal is to prevent disease worsening and hospitalization. Here, we are supporting data that sulodexide could be considered as an important therapeutic option for COVID-19.
Rationale
Endothelial cells can be infected by SARS-CoV-2: SARS-CoV-2 virus infects host cells through the Spike (S) protein that binds to the Angiotensin-Converting-Enzyme 2 (ACE-2) receptor, while the Type 2 Transmembrane Serine Protease (TMPRSS2) present in the host cells promotes viral uptake by cleaving ACE-2 and activating viral S protein [4]. ACE-2 receptor is expressed in several organs and cells, as type II alveolar cells, myocardial, proximal tubule of the kidneys, ileum, and bladder epithelial cells among others [5,6]. Endothelial cells are also rich in ACE-2 receptor and can be infected by this virus. Viral particles have been found in endothelial cells of lungs and kidneys [7,8]. Moreover, human vascular structures from in vitro designed organoids have also been infected by SARS-CoV-2 [9].
Endothelial injury including glycocalyx damage
Viral infection of endothelial cells causes an endothelial injury that may be severe. This endotheliitis has been observed in postmortem reports [7,8]. High plasma levels of markers of endothelial cell activation and platelet activation as von Willebrand Factor (vWF) antigen, soluble thrombomodulin and soluble P selectin were found in COVID-19 patients and were associated with critical illness and death. SCD40L a marker of T cell activation, as well as Plasminogen Activator Inhibitor-1 (PAI-1), vWF activity and factor VIII activity plasma levels were higher in intensive care patients. D-Dimer and thrombin-antithrombin complexes were also higher in chronic diseases with endothelial dysfunction as diabetes and hypertension that might predispose to a further viral endothelial damage leading to platelet and leukocyte activation as well as abnormal anticoagulant and fibrinolytic mechanism [10].
Also, exploring sublingual microvessels with intravital microscopy by sidestream dark field imaging in patients with COVID-19, a marked reduction of the density of small capillaries and severe glycocalyx damage were observed especially in those patients with mechanical ventilation. In these subjects severity of COVID-19 was correlated with several markers of endothelial dysfunction, strikingly von Willebrand factor-cleaving protease, and Vascular Endothelial Growth Factor-A (VEGF-A) [11].
Hypercoagulability and thrombosis
Activation of coagulation, starting from endothelial damage, is related to the severity of the disease. Higher levels of D-dimer and fibrin degradation products, longer prothrombin time and activated thromboplastin time in non-surviving than in surviving patients with severe COVID-19 pneumonia have been shown in Wuhan, China. Furthermore, severe cases have been complicated by disseminated intravascular coagulation, 74% of the non-survivors met criteria of disseminated intravascular coagulation compared with 6% of survivors [12]. Global viscoelastic hemostatic assay (thromboelastography) parameters and blood parameters of hemostasis were consistent with a state of hypercoagulability [13]. Incidence of Venous Thromboembolism (VTE) was 25% (20/81) in patients with severe coronavirus pneumonia. Higher levels of D-dimer were predictive of VTE [14]. Superficial thrombophlebitis can also occur in patients with COVID-19 [15], and in purpuric lesions of the skin thrombogenic vasculopathy with C5-9 and C4d accumulation (evidence of complement activation) has been found [16]. The role of inflammation in deep venous thrombosis has been recently addressed [17].
Inflammation
Significant inflammation is present at the beginning in COVID-19 with elevated levels of IL-6, C reactive protein, high ESR, and high fibrinogen levels. Some patients appear to have a more pronounced inflammatory response to infection with SARS-CoV-2, such as seen with Systemic Inflammatory Response Syndrome (SIRS) or cytokine storm, which may explain more dramatic changes in coagulation tests results, including significantly elevated D-dimer. Activation of host defense systems results in subsequent activation of coagulation and thrombin generation through humoral and cellular amplification pathways, a term called thromboinflammation. In sepsis, coagulation is activated by the inflammatory response through several procoagulant pathways. Cytokines elevation also result in activated vascular endothelial injury with resultant prothrombotic properties [18].
The mechanisms that activate coagulation in SARS-CoV-2 infection appear to be linked to endothelial damage and inflammatory responses rather than specific properties of the virus.
The three main pathophysiological features of COVID-19 appear to be severe microvascular and endothelial disturbances (including glycocalyx), hypercoagulability and severe inflammation.
Sulodexide
Sulodexide is a glycosaminoglycan composed of two distinct fractions: 80% of fast moving heparin and 20% of dermatan sulfate. Sulodexide has polypharmaceutical actions including endothelial protection, anti-thrombotic, profibribolytic, and anti-inflammatory. Its therapeutic use is in vascular diseases, mainly in chronic venous disease, in prevention of recurrent venous thromboembolism, and in diabetic nephropathy [19-23].
Sulodexide protects glycocalyx and endothelial cells: Sulodexide endothelial protection includes glycocalyx and endothelial cells. Glycocalyx is a thin layer constituted by proteoglycans, glycosaminoglycans and glycoproteins that covers the endothelium of all the vessel walls. It has several important physiological functions [24,25] and If perturbed may worse endothelial dysfunction [26]. Endothelial glycocalyx is injured in acute respiratory distress syndrome induced in mice by endotoxemia. Protection against endothelial injury by accelerating glycocalyx synthesis, attenuates glycocalyx damage, decreases IL-6 levels and improves survival rate in animal models [27]. Sulodexide inhibits glycocalyx permeability disturbances and oxidative stress in experimental ischemia-reperfusion injury [28]. In cultured endothelial cells sulodexide restores glycocalyx barrier [29]. In a balloon-injury of carotid artery model in rats, intraperitoneal injections of sulodexide could reconstruct endothelial glycocalyx, increased endothelial nitric oxide synthase levels, attenuated endothelial hyperplasia and inhibited platelet aggregation, decreased expression of CD31 and Intercellular Adhesion Molecule-1 (ICAM-1), normalized osteopontin and Vascular Cell Adhesion Molecule-1 (VCAM-1), and prevented CD68 inflammatory infiltration of vascular wall [30]. It has been demonstrated in diabetic subjects with reduced dimensions of endothelial glycocalyx that sulodexide given by oral route restores the glycocalyx thickness [31].
In cultured endothelial cells exposed to high glucose concentration sulodexide suppresses inflammation phenotype decreasing Reactive Oxygen Species (ROS), Monocyte Chemoattracting Protein-1 (MCP-1) and interleukin-6 (IL-6) and also increases the speed of wound repair of the cells layer [32]. A similar study with experimental human endothelial aged cells showed that sulodexide reduces senescence-related changes [33]. Sulodexide improves endothelial dysfunction in streptozotocin -induced diabetes in rats, while in cultered human umbilical vein endothelial cells, sulodexide counteracts inflammation and endothelial dysfunction induced by serum of patients with advanced chronic venous disease, or by metabolic stress (methylglyoxal) or irradiation. In these studies, cytoprotective effects of sulodexide resulted in a reduction of ROS production, a diminished synthesis and release of pro-inflammatory cytokines as IL-1, IL-6, IL-18, tumor necrosis factor-α (TNF-α), MCP-1, ICAM-1, and DNA damage [34-36]. Sulodexide also prevents apoptosis of endothelial cells exposed to oxygen-glucose deprivation. This protective effect seems to be mediated by reduced oxidative stress [37].
Sulodexide as anti-thrombotic: Sulodexide shows antithrombotic effects on platelet aggregation, plasma coagulation and fibrinolysis. It inhibits platelet aggregation in response to cathepsin G, thrombin and tissue factor. Counteracts plasma coagulation factors Xa and IIa, thus showing an anti-thrombin effect similar in vitro to enoxaparin [38-40]. Thrombolytic activity has been initially shown adding sulodexide to thrombi formed 6 hours before [41]. Sulodexide administered by oral route decreases Plasminogen Activator Inhibitor-1 (PAI-1) and enhances Tissue Plasminogen Activator (tPA) [42,43]. Antithrombotic activity of sulodexide given by oral route has been confirmed in clinical studies that have shown its efficacy for the prevention of recurrent venous thromboembolic episodes [44,45].
Sulodexide shows anti-inflammatory effects: Anti-inflammatory effects of sulodexide have been demonstrated in several studies in vitro, in animal models and in human beings. Sulodexide acts on regulatory inflammatory response decreasing mediators of inflammation as interleukins (IL) IL-6, IL-8, IL-1b, IL-2, IL-10, IL-13, interferon β, Macrophage Inflammatory Protein-1β (MIP-1β), Transforming Growth Factor-β1 (TGF-β1), Vascular Endothelial Growth Factor (VEGF), Monocyte Chemoatracttant Protein-1 (MCP-1), Tumor Necrosis Factor-α (TNF-α), granulocyte colony stimulating factor and granulocyte-monocyte colony stimulating factor. Sulodexide decreases oxidative stress by reducing Reactive Oxygen Species (ROS) and enhancing Super Oxide Dismutase (SOD), and diminishing metalloproteinase-9 which can cause tissue damage [46-48].
Sulodexide Is safe: Remarkably, sulodexide lacks severe adverse effects. Long term treatment (two years) did not show more bleeding cases than placebo. Furthermore, in meta-analysis showed less cardiovascular and total mortality than comparators: Direct oral anticoagulants, vitamin K antagonists, aspirin and placebo [49,50].
Conclusion
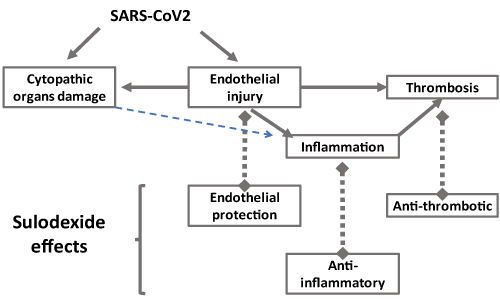
Endothelial damage, inflammation and thrombotic trend are important pathophysiologic features in COVID-19. Among therapeutic resources to address thromboinflammation in COVID-19 [51], sulodexide should be considered an important agent in patients with mild or severe COVID-19, because it acts against the major components of COVID-19 pathophysiology (Figure 1). Clinical investigation is warranted.
Figure 1: Vascular changes in COVID-19, endothelial injury, inflammation and thrombosis may be attenuated with sulodexide effects as endothelial protection, anti-thrombotic and anti-inflammatory activities.
Disclosure
Frati, Lecuona and Bautista are employees of Alfasigma Laboratories Mexico. Flores has been speaker for Alfasigma Laboratories and Servier Laboratories.
References
- Wiersinga WJ, Rhodes A, Cheng AC, PeacockSJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). A review. JAMA. 2020.
- Gupta A, Medhavan MU, Sehgal K, Nair N. Extrapulmonary manifestations of COVI-19. Nat Med. 2020.
- Horby P, Lim WS, Emberson J, Mahfam M, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVI-19: preliminary report. N Engl J Med. 2020.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herder T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181: 271-880.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navi GJ, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203: 631-637.
- Zou X, Chen K, Zou J, Han P, Hao J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020; 14: 185-192.
- Ackermann M, Verleden SE, Kuehmel M, Haverich A, Welte T, et al. Pulmonary vascular endothelialitis, thrombosis and angiogénesis in Covid-19. N Eng J Med. 2020; 383: 120-128.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395: 1417-1418.
- Monteil VKH, Prado P, Hagelkruys A. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020; 181: 905-913.
- Alvarado-Moreno JA, Majluf-Cruz A. COVID-19 and dysfunctional endothelium: The Mexican scenario. Arch Med Res. 2020; 51: 587-588.
- Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis. 2020.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18: 844-7
- Panigada M, Bottino V, Tagliabue P, Grosselli G, Novembrino C, et al. Hypercoagulability of COVID-19 patients in intesive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020; 18: 1421-1424.
- Demirbas A, Elmas OF, Tursen U, Atasoy M, Lotti T. Superficial thrombophlebitis in a patient with COVID-19: heparin treatment after evaluation of D-Dimer. Dermatol Ther. 2020.
- Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, et al. Complement activated injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020; 2020: 1-13.
- Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target ? Hematology. 2019; 24: 742-750.
- Connors J, Levy J. COVID-19 and its implication for thrombosis and anticoagulation. Blood. 2020; 135: 2033-2040.
- Hoppensteadt DA, Fareed J. Pharmacological profile of sulodexide. Int Angiol. 2014; 33: 229-235.
- Bignamini AA, Matuska J. Sulodexide for the symptoms and signs of chronic venous disease: A systematic review and meta-analysis. Adv Ther. 2020; 37: 1013-1033.
- Gaddi AV, Capello F, Gheorghe-Fronea O, Fadda S, Darabont RO. Sulodexide improves pain-free walking distance in patients with lower extremity peripheral arterial disease: a systematic review and meta-analysis. JRSM Cardiovasc Dis. 2020; 9: 1-14.
- Jiang QJ, Bai J, Jin J, Shi J, Qu L. Sulodexide for secondary prevention of recurrent venous thromboembolism: A systematic review and meta-analysis. Front Pharmacol. 2018; 9: 876.
- Frati-Munari AC. La sulodexida disminuye la albuminuria en pacientes con nefropatia diabética. Med Int Mex. 2020; 36: 530-542.
- Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflungers Arch. 2007; 454: 345-359.
- Frati-Munari AC. Medical significance of endothelial glycocalyx. Arch Cardiol Mex. 2013; 83: 303-312.
- Zhang X, Sun D, Song JW, Zullo J, Lipphardt M, et al. Endothelial cell dysfunction and glycocalyx- A vicious circle. Matrix Biol. 2018; 421-431.
- Suzuki K, Okada H, Takemura G, Takada C, Tomita H, et al. Recombinant thrombomodulin protects against lipopolysaccharide-induced acute respiratory distress syndrome via preservation of pulmonary endothelial glycocalyx. Br J Pharmacol. 2020; 177: 4021-4033.
- Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006; 290: H2247-H2256.
- Broekhuisen LN, Meuwese MC, Mooij HL, et al. Sulfated glycosaminoglycans restore glycocalyx barrier of cultured endothelial cells under hyperglycemic conditions. In: Meuwese MC, editor. Targeting the vessel wall in cardiovascular prevention. Amsterdam: Univ Amsterdam. 2008. 22: 46-54.
- Li T, Liu X, Zhao Z, Ni L, Liu C. Sulodexide recovers endothelial function through reconstructing glycocalyx in the balloon-injury in carotid artery model. Oncotarget. 2017; 8: 91350-91361.
- Broekhuisen LN, Lemkes BA, Monij HL, Meuwese MC, Verberne H, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with diabetes mellitus. Diabetologia. 2014; 53: 2646-2655.
- Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res. 2009; 153: 118-123.
- Suminska-Jasinska K, Polubinska A, Ciszewicz M, Mikstaki A, Antoniewicz A, et al. Sulodexide reduces senescence- related changes in human endothelial cells. Med Sci Monit. 2011; 17: 222-226.
- Urbanek T, Zbigniev K, Begier-Krasinska B, Baum E, Breborowicz A. Sulodexide suppresses inflammation in patients with chronic venous insufficiency. Int Angiol. 2015; 34: 589-596.
- Urbanek T, Kransinski Z, Suminska-Jasinska K, Baum E, Borej-Nowicka G, et al. Sulodexide reduces inflammatory reaction and senescence of endothelial cells in conditions involving chronic venous disease. Int Angiol. 2016; 35: 140-147.
- De Felice F, Megiorni F, Pietrantoni I, Tini P, Lessiani G, et al. Sulodexide counteracts endoithelial dysfunction induced by metabolic or non-metabolic stresses through activation of the autophagic program. Eur Rev Med Pharmacol Sci. 2019; 23: 2669-2680.
- Gabryel B, Jarzabek K, Machnik G, Adamczyk J, Belowski D, et al. Superoxide dismutase-1 and glutathione-peroxidase 1 are involved in the protective effect of sulodexide on vascular endothelial cells exposed to oxygen-glucose deprivation. Microvasc Res. 2016; 103: 26-35.
- Rajtar G, Marchi E, De Gaetano G, Cerletti C. Effects of glycosaminoglycans on platelet and leukocyte function: role of N-sulfation. Biochem Pharmacol. 1993; 46: 958-960.
- Adiguzel C, Iqbal O, Hoppensteadt D, Jeske W, Cunanan J, et al. Comparative anticoagulant and platelet modulatory effects of enoxaparin and sulodexide. Clin Appl Thromb Hemost. 2009; 15: 501-511.
- Cosmi B, Cini M, Legnani C, Pancani E, Calanni F, et. al. Additive thrombin inhibition by fast moving heparin and dermatan sulphate explains the anticoagulant effect of sulodexide, a natural mixture of glycosaminoglycans. Thromb Res. 2003; 109: 333-339.
- Barbanti M, Guizzardi S, Calanni F, Marchi E, Babbini M. Antithrombotic and thrombolytic activity of sulodexide in rats. Int J Clin Lab Res. 1992; 92: 179-184.
- Mauro M, Ferrero G, Palmieri G. Profibrinolytic and antithrombotic effects of sulodexide oral administration: a double blind, crossover, placebo controlled study. Curr Ther Res. 1992; 51: 342-350.
- Crepaldi G, Fellin R, Calabro A, Rossi A, Ventura A, et. al. Double-blind multicenter trial on a new medium molecular weight glycosaminoglycan. Current therapeutic effects and perspectives for clinical use. Atherosclerosis. 1990; 81: 233-243.
- Errichi BM, Cesarone MR, Belcaro G, Marinucci R, Ricci A, et al. Prevention of recurrent Deep venous thrombosis with sulodexide: the SanVal registry. Angiology. 2004; 55: 243-249.
- Andreozzi GM, Bignamini AA, Davi G, Palareti G, Matuska J, et al. Sulodexide for the prevention of recurrent venous thromboembolism: The sulodexide in secondary prevention of recurrent deep venous thrombosis (SURVET) study: A multicenter, randomized, double-blind, placebo-controlled study. Circulation. 2015; 139: 1891-1897.
- Mattana P, MannelloF, Ferrari P, Agus GB. Vascular pathologies and inflammation: the anti-inflammatory properties of sulodexide. J Vasc Endovasc Surg. 2012; 19: 1-7.
- Mannello F, Ligi D, Canale M, Raffetto JD. Sulodexide down-regulates the release of cytokines, chemokines, and leukocyte colony stimulating factors from human macrophages: role of glycosaminoglycans in inflammatory pathways of chronic venous disease. Curr Vasc Pharmacol. 2014; 12: 173-185.
- Munari ACF, Cervera LFF. Inflammation, metalloproteinases, chronic venous disease and sulodexide. J Cardiovasc Dis Diag. 2015; 3: 203.
- Bikdeli B, Chatterjee S, Kirtane AJ, Parikh SA, Andreozzi GM, et al. Sulodexide versus control and the risk of thrombotic and hemorrhagic events: metaanalysis of randomized trials. Semin Thromb Hemost. 2020.
- Pompilio G, Integlia D, Raffetto J, Palareti G. Comparative efficacy and safety of sulodexide and other extended anticoagulation treatments for prevention of recurrent venous thromboembolism: a Bayesian network meta-analysis. TH Open. 2020; 4: e80-e93.
- Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, et al. Pharmacological agents targeting thromboinflammation in COVID-19. Review and implication for future research. Thromb Hemost. 2020; 120: 1004-1024.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.