Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case report
- |
- Open Access
- |
- ISSN: 2639-4383
Evaluation of Valvular Hemodynamics After Surgical Aortic Valve Replacement and Their Impact on the Postprocedural Result After Transcatheter Valve in Valve Implantation
- Philipp Kiefer*;
- Department of Cardiac Surgery at Heart Center Leipzig, University of Leipzig, Saxony, Germany.
- Alexandro Hoyer;
- Department of Cardiology at Heart Center Dresden, University of Dresden, Saxony, Germany.
- Thilo Noack;
- Department of Cardiac Surgery at Heart Center Leipzig, University of Leipzig, Saxony, Germany.
- Jörg Seeburger;
- Department of Cardiac Surgery at Heart Center Leipzig, University of Leipzig, Saxony, Germany.
- Norman Mangner;
- Department of Cardiology at Heart Center Dresden, University of Dresden, Saxony, Germany.
- Axel Linke;
- Department of Cardiology at Heart Center Dresden, University of Dresden, Saxony, Germany.
- Felix Woitek;
- Department of Cardiology at Heart Center Dresden, University of Dresden, Saxony, Germany.
- Michael Borger;
- Department of Cardiac Surgery at Heart Center Leipzig, University of Leipzig, Saxony, Germany.
- David Holzhey
- Department of Cardiac Surgery at Heart Center Leipzig, University of Leipzig, Saxony, Germany.

| Received | : | Nov 28, 2020 |
| Accepted | : | Dec 24, 2020 |
| Published Online | : | Dec 30, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Kiefer P, Hoyer A, Noack T, Seeburger J, Mangner N, Linke A, et al. Evaluation of Valvular Hemodynamics After Surgical Aortic Valve Replacement and Their Impact on The Postprocedural Result After Transcatheter Valve in Valve Implantation. Ann Cardiol Vasc Med. 2020: 3(1); 1037.
Abstract
Background: The aim of this study was to determine the influence of the initial hemodynamics after Surgical Aortic Valve Replacement (SAVR) on the postprocedural outcome after transcatheter Valve-in-Valve implantation (ViV).
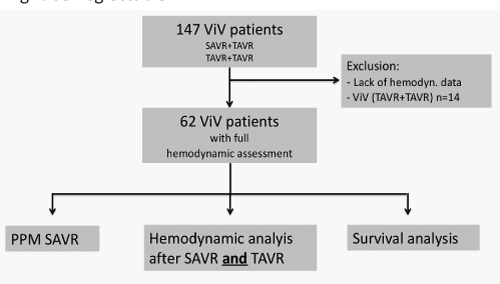
Methods: 147 patients received ViV due to failed surgical aortic valve bioprosthesis between 01/2008 and 5/2019. Aortic valve gradients after SAVR and after transcatheter ViV implantation were available in 62 patients (42.2%). Sizes of the previous surgical valves were characterized as small (≤21 mm, n= 14), intermediate (>21 - < 25 mm, n= 30) and large (≥ 25 mm, n= 17). Follow up was closed in 05/19.
Results: Mean age was 77.4 ± 7.3 years (59.6% female). Mean log EuroScore1 was 27.4 ± 16.4%. Stenosis was the leading pathology in 80.6% (mean Pmax: 65.5 ± 28.1 mmHg; mean effective orifice area [EOA]: 0.69 ± 0.2). The operative approach was transfemoral in 72.5% (n=45), transapical in 22.5% (n= 14) and direct aortic in 4.8% (n=3) of the patients. In 30 patients (48.3%) a self-expandable Transcatheter Heart Valve (THV) and in 32 patients (51.6%) a balloon-expandable THV was implanted. 30-day mortality was 6.5% (4/62). Patients with small label sizes, transvalvular gradients (Pmax/mean 30.8 ± 9.6/16.2 ± 5.8 mmHg) were not statistically different after ViV compared to the gradients after SAVR (35 ± 13.2/19.7 ± 8.9; p= 0.371/p= 0.331). For patients with intermediate label sizes, the Pmax gradient after ViV (35.7 ± 15.3 mmHg) was significantly higher compared to the starting gradient after SAVR (28.3 ± 9.0 mmHg, p= 0.035). Elevated transvalvular mean gradients were more often recorded after ViV in the intermediate group (small: 6/15, 40%; intermediate: 17/30, 56.7%; large: 2/17, 11.8%; p= 0.011).
Conclusions: ViV represents a good alternative for treatment of degenerated aortic bioprostheses, even for small sizes. However, individual decision making, concerning PPM with indexed EOA, initial hemodynamics after the SAVR and the label of the bioprosthesis, is necessary.
Introduction
The use of biological prostheses for Surgical Aortic Valve Replacement (SAVR) has increased recently [1,2]. Correspondingly the cut-off age for implantation of a bioprosthesis, despite of the guidelines, has been consequently reduced during the last decades [3]. The improvement of durability of bioprostheses and the patients demand due to avoidance of life-long anticoagulation might justify this paradigm shift. But also the possibility of a transcatheter aortic valve replacement (TAVR) as a Valve-in-Valve (ViV) procedure in order to circumvent a surgical re-do procedure. However, an increased number of patients with a degenerated bioprosthesis will have to undergo treatment in the future.
Recently, the Global Valve-in-Valve Registry (VIVID) has reported good 1-year survival for selected patients undergoing ViV [4]. In a consecutive analysis of the VIVID cohort, Pibarot and colleagues have reported that the occurrence of Prosthesis-Patient Mismatch (PPM) is negatively impacting on early postoperative and one-year survival [5]. Therefore, this study was designed to evaluate the influence of the initial hemodynamics of the biological prostheses after SAVR on the procedural success of the ViV procedure. To the best of our knowledge, there is no study evaluating the hemodynamic function after the initial SAVR procedure in relation to the hemodynamic result after ViV.
Patients and methods
Overall 147 patients, who received transcatheter aortic valve-in-valve implantation due to failed surgical aortic valve prostheses, were identified after review of our institutional data board. All patients received a ViV via a transfemoral, transapical or transaortic approach between January 2008 and May 2019. For 62 patients (42.2%), complete echocardiographic data sets after the initial surgical aortic valve implantation, and pre-/post ViV implantation were available. Patients with insufficient data quality were excluded from further analysis (Figure 1 & Table 1), mostly because initial SAVR was not performed in our center and postprocedural hemodynamics could not be found out.
Patients were followed by the referring cardiologist and contacted periodically by our own study team, either through outpatient visit and telephone contact with patients, family members, or both. Supplemental information was provided from family physicians and referring cardiologists. Our study team collected all follow-up data. Follow up was 87% complete and closed in May 2020.
This study was approved by the ethics committee (registration no. 428/17-ek) and was performed according to the Declaration of Helsinki. Written informed consent was provided by all patients who were in an adequate conscious state preoperatively.
Definitions
Risk stratifications were calculated for each patient using the Society of Thoracic Surgeons (STS) score and the log EuroSCORE1. The definition of a structural valve degeneration of surgical bioprosthetic aortic valves was adapted from Dvir et al [6]. Post-implantation hemodynamic data were derived from the first postprocedural echocardiography after the initial surgical AVR and the ViV procedure. Body Surface Area (BSA) was calculated with the Mosteller formula. Prosthesis-patient mismatch, which is calculated by the divide of the EOA of the prosthetic valve and the BSA of the patient, is defined as severe when less than 0.65 cm2/m2, mild/moderate between 0.65-0.85 cm2/m2 and none when greater than 0.85cm2/m2. Sizes of the previous surgical valves were characterized as small (label size ≤21 mm, n= 14), intermediate (>21 mm and < 25 mm, n= 30) and large (≥ 25 mm, n= 17). Vascular complication, bleeding and kidney injury were defined in accordance with the Valve Academic Research Consortium-2 (VARC-2) criteria [7].
Implantation technique and Valve Sizing
Each patient was evaluated in the Heart Team before intervention. If the operative risk was deemed high due to comorbidities or age, patients were considered for a ViV procedure. The standard approach for the intervention was transfemoral. Only when there were contraindications like pronounced peripheral artery disease prohibiting transfemoral access, a transapical or transaortic approach was considered. Valve sizing was derived from the internal diameter of the surgical valve as indicated from the label size and manufacturer charts [8]. Even though label size was known, internal diameters were additionally calculated by following imaging modes such as computed tomography and transesophageal echocardiography and were also be taken into consideration [9].
Statistical analysis
Continuous variables were expressed as mean and standard deviation. Categorical variables were expressed as absolute numbers and percentages. Differences between groups were analyzed using the student t- test and the Fishers exact test for comparison of two groups as appropriate. For comparison of three or more groups ANOVA was used. Hospital mortality was defined as death in house or within 30 days post discharge. Kaplan-Meier survival curves were calculated and tested by log rank to describe the differences in survival. All analyses were performed with Sigma plot (Sigma Plot 12.5, Germany). P-values <0.05 were considered to be statistically significant. The data were reported according to the “Guidelines for Reporting Mortality and Morbidity After Cardiac Valve Operations” by Akins and colleagues [10].
Results
Patient demographics
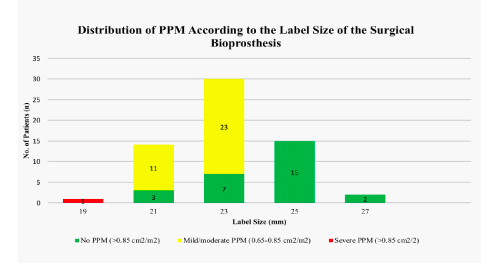
The clinical characteristics of the study cohort are shown in Table 2. Mean age was 77.4 ± 7.3 (range 51-88) and 59.6% were male. The majority of patients (80.6%) were in New York Heart Association (NYHA) functional class III or IV. The operative risk, as expressed by log EuroSCORE 1, was 27.4 ± 16.4 and 9.1 ± 5.4 calculated by STS-Score. Mean interval time between the initial SAVR and ViV procedure was 9 ± 3 years. The leading mechanism of valve failure was stenosis in 80.6%, regurgitation in 14.5% and a combined lesion in 9.6%. Severe PPM was present in one patient (1.6%) with a bioprosthesis with label size 19 mm. Mild/moderate PPM was seen in 11 patients (78.6%) with label size 21 mm, in 22 patients (75.7%) with label size 23 mm and no PPM was calculated in patients with prostheses >23 mm (Figure 2). Transvalvular gradients were significantly higher after SAVR with small and intermediate label sizes (Pmax small: 30.8 ± 9.9 mmH, Pmax intermediate: 28.2 ± 9.2 mmHg) compared to large label sizes (Pmax: 21.2 ± 1.9 mmHg, p=0.006).
Procedural results
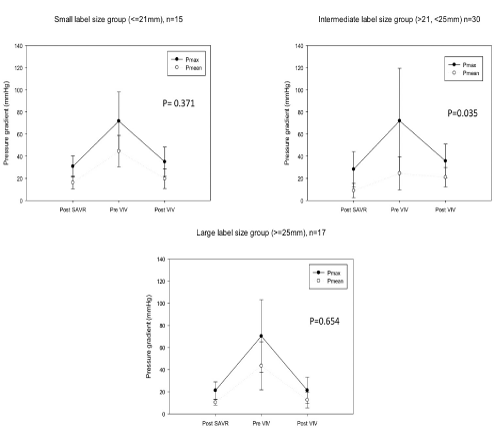
The periprocedural results are depicted in Table 3. The operative approach was transfemoral in 72,5% (n= 45), transapical in 22.5% (n= 14) and transaortic in 4.8% (n= 3). The mode of bioprosthesis failure was not different between the groups with small (stenosis: n= 12, 80%; regurgitation: n= 3, 20.0%; combined: 0), intermediate (stenosis: n= 24, 80%; regurgitation: n= 3, 10%; combined: n= 3, 10%) and large label sizes (stenosis: n= 14, 82.4%; regurgitation: n= 3, 17.6%; combined: 0). There were no significant differences in the use of Self-expandable or Balloon-expandable Transcatheter Heart Valves (THV) for patients with small (Self-expandable: n= 8, 57.1%; Balloon-expandable: n= 6, 42.9%, p= 0.706), intermediate (Self-expandable: n= 13, 43.3%; Balloon-expandable: n= 17, 56.7%, p= 0.438) and large THV (Self-expandable: n=10, 58.8%; Balloon-expandable: n=7, 41.2%, p= 0.439). Transvalvular gradients after the initial operation, before and after the ViV procedure are shown in Figure 3. Postprocedural gradients could be reduced in all patients. For patients with small label sizes transvalvular gradients were not statistically different after ViV compared to the gradients after SAVR (Table 4). For patients with intermediate label sizes the Pmax gradient after ViV was significantly higher compared to the starting gradient after SAVR (28.3 ± 9.0 mmHg and 35.7 ± 15.3 mmHg, p= 0.035). Pmean after the initial SAVR and after ViV did not reach statistical significance (15.9 ± 6.6 mmHg and 21.0 ± 8.7, p= 0.069). The postprocedural Effective Orifice Area (EOA) was greatest in the group with large label size prostheses and significantly greater compared to the small group (large: 1.8 ± 0.5 cm2; small: 1.2 ± 0.3 cm2, p= 0.041). Elevated transvalvular mean gradients were more often recorded after ViV in the intermediate group (small: 6/15, 40%; intermediate: 17/30, 56.7%; large: 2/17, 11.8%; p= 0.011). In 12.9% (8/62) of the patients a mild and in one (1.6%) patient a moderate paravalvular regurgitation was detected after the procedure.
Postoperative outcome
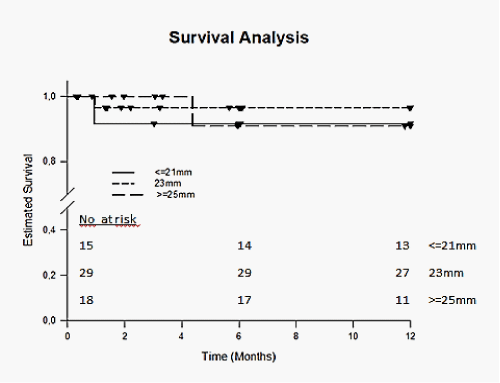
The early postprocedural outcome is shown in Table 5. Mean hospital stay after ViV was 12.1 ± 7.2 days for all patients and was not different between the groups. Immediate mortality within 72 hours of the procedure was 3.2% (2/62) and overall 30-day mortality was 6.5% (4/62). The cause of death was cardiovascular in 37.5% (3/8). Major stroke occurred in 2 patients (3.2%). Bleeding complications were observed in 6.5% (4/62) patients and major vascular complications were observed in 8.1% (5/62). Acute kidney injury (AKIN classification) stage 2 or 3 was seen in 3.2% (2/62). A permanent pace-maker implantation following the procedure was necessary in 6.5 % (4/62). Kaplan-Meier analysis revealed no significant differences in 1-year survival for patients with small, intermediate and large label sizes (p= 0.777) and between patients treated with self-expandable or balloon-expandable THV (p= 0.443) (Figure 4).
Study limitations
Due to the retrospective nature of the study the endpoints were not defined prospectively and therefore not all important echocardiographic parameters such as EOA were available in all patients after SAVR and ViV. Another bias might be due to the fact that most of the ViV procedures were conducted in patients with intermediate label size bioprostheses and thus statistical comparability is limited. Furthermore, due to the small study-population a differentiation of the different surgical prostheses and their influence on the postprocedural outcome might be misleading. However, the vast majority of patients have had a Perimount (Edwards Lifesciences, Irvine, CA, USA) or Epic (St. Jude, St. Paul, MN, USA) bioprosthesis and therefore this bias might be neglectable.
Figure 3: Depiction of transvalvular gradients (pmax/mean) after SAVR, before ViV and following ViV for the different label size groups.
Table 1: Proportion of the excluded patient data (N= 85) by initially implanted bioprosthetic valve size.
Table 4: Hemodynamic measurements after SAVR, before ViV and following ViV for the different label size groups.
Discussion
The upcoming of TAVR has substantially changed the treatment options for patients with heart valve diseases. Especially for patients presenting with degenerated bioprostheses, who normally present at a higher age and with higher operative risk, the ViV technique has been proven to be save and feasible [7,8]. However, some technical and procedural challenges such as PPM after ViV are of major concern [4]. Our single-center study was therefore designed to investigate the influence of the hemodynamics after the initial SAVR on the postprocedural outcome after ViV. As described before, we distinguished the different surgical valve label sizes in small (≤21 mm), intermediate (>21<25) and large (≥25) in order to detect any determination of the label size on the postprocedural result. In our cohort the majority of patients presented for a ViV procedure with a preexisting PPM. Patients with PPM were more likely to have higher gradients after the procedure. Interestingly, in patients with small label sizes a reduction of the gradients compared to the postsurgical hemodynamics was possible, whereas in patients with intermediate primarily label size 23 mm, we found elevated gradients after the ViV procedure.
Dvir et al. have reported the early and one-year results of the ViVid registry [3]. They described as main risk-factors for a worsened outcome after ViV: Small surgical valves, a preexisting stenosis and PPM. These findings are prima faciae not congruent with our results. But there might be some differences between both cohorts. First, in the VIVID cohort the preexisting mode of valve failure was equally distributed between stenosis, regurgitation and combined, whereas in our cohort the vast majority of patients presented with a stenosis as the leading mechanism of failure. Second, the PPM in patients with small and intermediate label sizes was only moderate while in the VIVID cohort the patients who presented with PPM were predominantly patients with severe PPM and small surgical bioprostheses (≤21 mm). Nevertheless, our results suggest that although a determination of the size of the THV during ViV is given by the implanted bioprostheses, an acceptable hemodynamic result can be achieved compared to the postoperative gradient after SAVR, also in relation to the Follow-up. It has been suggested that the use of balloon-expandable THV for ViV might be associated with higher gradients [3]. In our series there were no significant differences in the use of self-expandable versus balloon expandable valves regarding the different groups. However, in the intermediate label size group, there was a higher degree of balloon-expandable THV compared to the other groups. This might explain the worse outcome in hemodynamic measures in this group, whereas we could not detect a difference in clinical endpoints such as 30 day mortality.
Another reason for the higher gradients in the intermediate label size group could be, that the implantation depth of the THV during the ViV procedure might have been different compared to the other groups. It has been reported that a higher positioning of the THV improves the postprocedural hemodynamic result [11]. This finding has led to a shift of implantation depth towards a higher supraannular implantation during the last years in our institution. However, the distribution of the time of implantation as an indicator for the implantation depth was equally between the groups.
The increasing use of bioprostheses even in young patients (<60 years) will confront health care professionals with heart valve diseases due to degenerated surgical valves in the future [3]. Therefore, the ViV procedure will play a major role, because it can be conducted with excellent early postoperative survival and good postprocedural hemodynamic results. But not only the necessity of treating such patients, the promise of having these techniques already available to treat degenerated valves in the future, might trigger the use of bioprostheses even in younger patients. And the debate about the best treatment strategy, for example: First SAVR followed by Re-SAVR and then ViV, or first TAVR followed by SAVR and then ViV is going on. This will push the cut-off age for bioprostheses potentially down and hopefully improve the quality of life and longevity of the patients.
Conclusion
PPM after surgical aortic valve replacement is associated with a higher mortality rate, especially when TAVR as ViV is followed. Small label sized bioprostheses, especially those less or equal 21 mm, are discredited to have the worst outcome after ViV. However, little is known about the hemodynamics after the initial SAVR and its influence on the outcome for ViV in failed bioprostheses. In our study, we revealed, that an acceptable hemodynamic outcome after the initial AVR, regardless of none or moderate PPM, the hemodynamics after ViV in our series were not be significantly affected - and also not the outcome. However, more data and also randomized controlled trials are needed for proof.
We conclude, that assessing PPM for surgical AVR is of course a prerequisite. Size and label of bioprosthesis should be chosen critically for potential further interventions and optimal outcomes. Therefore, transcatheter valve implantation in a failed bioprostheses is a multifactorial complex procedure, that should not only be addressed by the indexed EOA.
References
- Isaacs AJ, Shuhaiber J, Salemi A, Isom OW, Sedrakyan A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg 2015; 149: 1262-1269.
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108 687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009; 137: 82-90.
- Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, Woo YJ. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. New England Journal of Medicine. 2017; 377: 1847-1857.
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter Aortic Valve Implantation in Failed Bioprosthetic Surgical Valves. JAMA 2014; 312: 162–170.
- Pibarot P, Simonato M, Barbanti M, et al. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC: Cardiovascular Interventions. 2018; 11: 133-141.
- Dvir D, Bourguignon T, Otto CM, et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation. 2018; 137: 388-399.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. The Journal of thoracic and cardiovascular surgery. 2013; 145: 6-23.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010; 121: 1848-1857.
- Gurvitch R, Webb JG, Yuan R, et al. Aortic annulus diameter determination by multidetector computed tomography: reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC: Cardiovascular Interventions. 2011; 4: 1235-1245.
- Akins CW, Miller DC, Turina, MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. The Journal of Thoracic and Cardiovascular Surgery. 2008; 135: 732-738.
- Simonato M, Webb J, Kornowski R, et al. Transcatheter replacement of failed bioprosthetic valves: large multicenter assessment of the effect of implantation depth on hemodynamics after aortic valve-in-valve. Circulation: Cardiovascular Interventions. 2016; 9: e003651.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.