Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Review Article
- |
- Open Access
- |
- ISSN: 2639-4383
Melatonin as a Novel Candidate for Gene Therapy of Atherosclerosis
- Amirhosein Moradi;
- School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- Abdolreza Esmaeilzadeh;
- Immunotherapy Research and Technology Group, Zanjan University of Medical Sciences, Zanjan, Iran
- Reza Elahi*
- School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran

| Received | : | Aug 02, 2020 |
| Accepted | : | Sep 09, 2020 |
| Published Online | : | Sep 11, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Moradi AH, Esmaeilzadeh A, Elahi R. Melatonin as a Novel Candidate for Gene Therapy of Atherosclerosis. Ann Cardiol Vasc Med. 2020: 3(1); 1027.
Keywords: Atherosclerosis; Gene therapy; Melatonin; Inflammation; Oxidative stress
Abstract
Cardiovascular diseases remain the main cause of death worldwide. Atherosclerosis, the chronic inflammation of large and medium vessels, is the main cause of cardiovascular diseases and their complications. Inflammation has proved to be involved as the main promoter of the atherosclerotic plaque progression. Also, oxidative stress is another factor that has a key role in the pathogenesis of atherosclerosis. Melatonin is a neuroendocrine hormone that is produced by multiple organs mainly by pineal gland. Exogenous administration of melatonin has recently shown atheroprotective effects. Athero-protective activities of melatonin are coducted by its anti-oxidant (free radical scavenging) and anti-inflammatory role in chronic inflammation. Melatonin exerts its anti-inflammatory effects through suppressing NLRP3 inflammasome and reducing expression of TLR-4 receptor, which is the upstream of NF-k β pathway. Suppression of the mentioned pathways reduces the production of inflammatory mediators and cytokines. It has also been shown that melatonin can stabilize the plaque by increasing the levels of collagen inside the atherosclerotic plaque. Melatonin is mostly synthesized in the pineal gland; however, lymphocytes also have the capacity to produce it, as well. Biosynthesize of melatonin include several enzymes. Two of them, AANAT and HIOMT, act as rate limiting enzymes. Regulatory T cells have shown to have an atheroprotective effect through their immune suppressive activity. According to melatonin producing capacity and atheroprotective effects, for the first time, in order to induce melatonin production, the authors propose the overexpression of AANAT in T regs as a novel candidate for gene therapy of atherosclerosis. Since melatonin is an endogenous molecule that has an athero-protective role with low adverse effects, the overexpression of AANAT gene in T reggs could result in atherosclerosis plaque regression and increase plaquestability.
Introduction
Cardio Vascular Disease (CVD) is the leading cause of mortality worldwide. Atherosclerosis (AS) is a chronic inflammation of the large and medium vessels which has been identified as the main cause of CVD. Thus, there is a significant necessity for developing AS-reducing strategies in order to restrict its severe complications, such as Myocardial Infarction (MI), Stroke, Unstable Angina (UA), and sudden cardiac death.
Atherosclerosis (AS)
Atherosclerosis plaque formation
AS plaque formation is the consequence of Low-Density Lipoprotein (LDL) deposit in the sub-endothelial layer of the large and medium vessels and the inflammatory responses to this deposition [1,2]. This process contains several stages. The initial step is identified by endothelial damage that creates a pathway for plasma LDL aggregation in the sub-endothelial layer [1,3]. Then, the deposited LDL is converted into oxidized (ox)-LDL by existing Reactive Oxygen Species (ROS) in the subendothelial layer [4]. Resident macrophages and recruited monocytes can uptakethe ox-LDL through their receptors [4,5]. Accumulation of the ox-LDL in macrophages leads to the formation of the foam cells. This process results in the production of various cytokines by foam cells. Also, ox-LDL activates macrophages and endothelial cells to produce chemokines and several adhesion molecules that facilitate monocyte recruitment to the sub-endothelial layer [1,4]. The ox-LDL iniside the foam cells and apoptotic cytokines accompany to induce the apoptosis of the foam cells [6]. This leads to formation of the macrophage debris in the subendothelial layer. Defective clearance (efferocytosis) of dead macrophages results in chronic inflammation and necrotic nucleus formation.Necrotic nucleus destabilizes the plaque and increases the risk of rupture and thrombosis that consequently lead to acute coronary syndrome including MI and UA [6].
Atherosclerosis and inflammation
Inflammation is the response of the immune cells to harmful stimuli, such as foreign pathogens. Inflammation has been validated to play a pivotal role in AS process [3,7]. The monocyte macrophage system is the most important immune component that participatesto AS plaque progression [8]. Ox-LDL causes the endothelial cells to produce chemotacticand adhesion molecules that result in the migration of the monocytes to the subendothelial layer. After monocytes migration, they differentiate into macrophages and start to uptake ox-LDL and form foam cells. Ox-LDL can also induce inflammatory responses through Toll-Like Receptor (TLR) and nuclear transcription factor-kappa B (NF-kB) in foam cells. This finallycauses pro-inflammatory cytokine secretion [1].
Uncontrolled uptake of ox-LDL by macrophages causes the production of inflammatory mediators by activating NF-kB transcription factor. This consequently ends in chronic inflammation. Cytokines are the major mediators of inflammation that participate in all stages of the AS. They could be divided into two main groups: Pro-inflammatory and anti-inflammatory cytokines. Pro-inflammatory cytokines, including Inter Leukin-1 (IL-1), 6, 12, and, 18 and TNF-alpha, have shown to promote the progression of AS. Anti-inflammatory cytokines including IL-5, 10 and 13 have shown to havean inhibitory role in AS plaque progression [2].
Different kinds of immune cells have been detected to be involved in AS development. In this process, monocyte/macrophage system plays a major role. Moreover, T cells have a significant role in AS development, as well [8]. In conclusion, both innate and adaptive immune cells play a pivotal role in AS plaque progression [3,9].
T cells and atherosclerosis
Different types of T cells, including T helper 1 (Th1), Th2, and regulatory T cells, are involved in AS development. Th1 is known as a pro-atherogenic cell due to its pro-inflammatory activities. The role of Th2 in AS peogression is controversial. IL-4-deficiency, a Th2 related cytokine, has been shown to decrease A Splaque formation. On the other hand, IL-10, which is another Th2 related cytokine, has shown a powerful anti-atherogenic effect. Regulatory T cells (T regs) have two subtypes: Natural T regs and induced T regs. Studies have demonstrated that both types of T regs have powerful athero-protective activity due to their immune-suppressing activity [3,10].
Oxidative stress and AS
Oxidative stress plays a key role in AS plaque development. Oxidation of the deposited LDL leads to the production of ox-LDL. Ox-LDL is uptaken by the macrophages that leads to formation of the foam cells [11]. Since NF-kB is sensitive to oxidative stress, it can be activated by ox-LDL [12]. Also, ox-LDL induces NLRP3 (nucleotide-binding domain and leucine-rich repeat pyrin domain containing 3) inflammasome that inducesIL-1β and IL-18 secretion [13]. Also, the activation of the macrophage inracellular pathways by ox-LDL results in the production of inflammatory mediators [4], such as Monocyte Chemo attractant Protein-1 (MCP-1) [14], Vascular Cell Adhesion Molecule-1 (VCAM-1) [15],TNF-a [16], IL-6, and IL-8 [17,18]. Ox-LDL can induce the apoptosis of the Smooth Muscle Cell and macrophages which ends in instability of plaque. In conclusion, the induction of proinflammatory mediators by ox-LDL leads to chronic inflammation [11], that causes AS plaque progression and instability. Thus, anti-oxidant agents could be beneficialin inhibiting AS plaque progression [6,15,19].
Novel approaches to inhibit the progression of AS plaque
Recently, the standard of care for AS is based on reducing the CVD risk factors, including smoking, high serum LDL, diabetic mellitus, obesity, and hypertension. LDL-lowering drugs, such as statins, Proprotein Convertase Subtilisin/kexin type 9 (PCSK9) inhibitors, and also some other anti-IL1β monoclonal Abs are also employed [20-22]. HMG-CoA reductase inhibitor (statins), Phospholipase A2 (PLA2) inhibitors, and 5-Lipooxygenase inhibitors (LOX inhibitors) ameliorate atherosclerotic plaque by their anti-inflammatory effects [23]. However, in addition to anti-inflammatory agents, anti-oxidants have also shown atheroprotective effects. Although these drugs have shown acceptable effects in reducing serum LDL levels and inhibiting the progression of therosclerosis, there is still a strong demand for designing novel anti-inflammatory and anti-osxidant methods with higher efficacy. Also, statins can not appropriately control the progression of AS plaque [1].
Gene therapy has recently shown promising results in inhibiting the progression of atherosclerosis. Gene therapies follow two purposes in AS treatment: 1) gene replacement therapy, 2) over-expression of some molecules or proteins that inhibit atherosclerosis or stabilize AS plaque [24]. Gene replacement therapy of familial hypercholestrelemia, an autosomal codominant disorder that causes elevation in plasma LDL, results in AS plaque regression. Sadik H. Kassim et al. studied the utilization of liver-specific adeno-associated virus serotype 8 (AAV-8) vectors coding human cDNA of LDL receptor in humanized mouse model of familial hypercholesterolemia. This study exhibited promising effects in lowering plasma LDL [25]. IL-10 has an anti-inflammatory role in AS. Paul L. Hermonat et al. showed that gene transferring of IL-10 using Adeno-Associated virus 2 (AAV2) vector significantly inhibited the development of AS in LDL-R-deficient mice [26]. Since each drug or method may have adverse effects, developing new inflammation-reducing approaches with low adverse effects could show more efficacy against AS Thus, selecting an anti-inflammatory agent or molecule with low adverse effect and endogenous origination may manifest superior prominence as a candidate for gene therapy of AS. Studies show that melatonin is a molecule that has anti-inflammatory and anti-oxidant potency with an endogenous origination. Thus,melatonin may be a good candidate for gene therapy of AS.
Melatonin
Melatonin and its biosynthesize
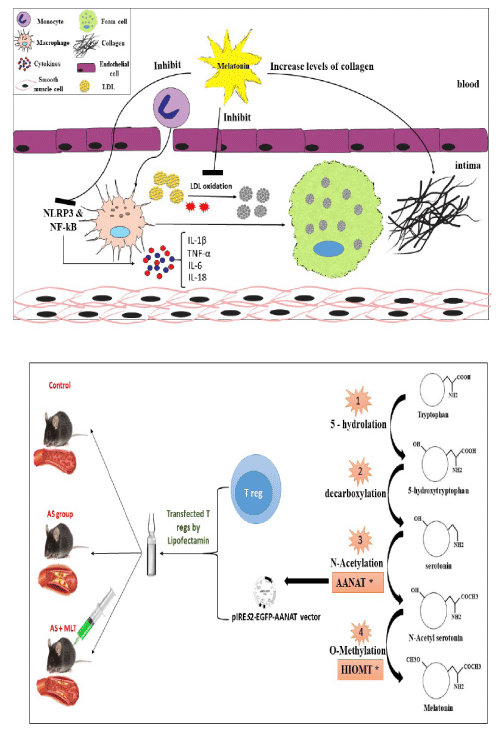
Melatonin is a neuroendocrine hormone that is mainly produced by the pineal gland. However, evidence show that some extra-pineal tissues, such as astrocytes, glial cells, lymphocytes, retinal cells, testes, ovary, placenta, and skin, also have the capacity to biosynthesize melatonin. Melatonin is one of the tryptophan’s derivatives and its synthesizes includes four steps. Cascade of melatonin production summurized in figure 1. There are two rate-limiting enzymes: Arylalkylamine N-acetyltransferase (AA-NAT) and hydroxyindole O-methyl transferase (HIOMT). Thus, overexpression of AANAT and HIOMT could lead to increased melatonin production.
Melatonin can exert its effects directly or through it receptor-mediated pathways. Receptor-mediated responses are mediated by two kinds of receptors. Membrane receptors: Melatonin receptor 1(MT1) and MT2 in human, and nuclear receptors, that are subfamilies of Retinoid Orphan Receptor/Retinoid Z Receptor (ROR/RZR). Previously, melatonin was known for its circadian effect; however, it also has known to have an anti-oxidant and anti-inflammatory role, as well. These roles will be briefly explained in the next parts [27-29].
Melatonin and inflammation
Chronic inflammation plays a key role in the immunopathogenesis of AS. Thus, anti-inflammatory agents could be considered as a beneficial method for preventing AS. Melatonin has shown both pro and anti-inflammatory effects, which means it could be both activator and inhibitor of the inflammation. Studies show that melatonin plays a pro-inflammatory role in the early stages of the inflammation/acute inflammation; however, it can act as an anti-inflammatory agent in chronic inflammation. As mentioned, TLR-4 and NF-kβ interaction result in the production of pro-inflammatory cytokines, including IL-12 and MCP-1. It has been demonstrated that Melatonin inhibits the expression of TLR-4-mediated inflammatory genes through the MyD88 dependent pathway [30]. It has been also reported that melatonin inhibits the activation of nucleotide-binding oligomerization domain-like receptor pyrin domain 3 (NLRP3 inflammasome) which results in reduced caspase-1, and consequently, low levels of IL-1β and IL-18 [12]. The anti-inflammatory effect of melatonin is also conducted through down-regulation of inflammatory mediators such as PLA2, LOX, IL-1, and TNF-α. In conclusion, melatonin acts as an anti-inflammatory agent in chronic inflammation through the down-regulation of pro-inflammatory mediators [27].
Li et al. studied the in-vitro effect of melatonin on inflammation of the smooth muscle cell and atherosclerosis in apoE-/- mice. This study revealed that melatonin reduced smooth mucle cell inflammation by inhibiting the production of TNF-α and Platelete Derived Growth Factor-BB (PDGF-BB). Melatonin also reduced the in-vitro formation of foam cells in ox-LDL treated macrophages [31].
Autophagy is the process that involves digestion of intracellular organelles and helps cells to survive against stressful condition [32]. Studies have demonstrated that autophagy results in progression of inflammation and atherosclerosis, in late stages of the atherosclerosis. Thus, targeting autophagy could act as promising method in treatment of atherosclerosis [32,33]. Oxidative stress is one of the main factors that can trigger autophagy. Since melatonin is an anti-oxidant agent, it can regulate the autophagy through its anti-oxidative capacity. However it is not the only mechanism for melatonin which could regulate the autophagy, and further studies are required to investigate mechanisms of melatonin-regulated autophagy inhibition [32,34].
Melatonin and oxidative stress
In addition to melatonin’s anti-inflammatory effect, it is also an anti-oxidant molecule. Its antioxidant activity is related to its free radical scavenging effect and regulatory role in anti-oxidant enzyme expression. As mentioned, nuclear transcription factor-kappa B (NF-κB) is an oxidative stress-sensitive transcription factor that plays a pivotal role in inflammation [12]. Sine melatonin can reduce the oxidative stress, application of melatonin leads to the down-regulation of NF-κB and can consequently reduce inflammation. Studies also show that melatonin decreases NO production in inflamed tissue by down-regulation of Nitric Oxide Synthase (NOS) through NF-κB pathway. In conclusion, melatonin could be considered as an antioxidant agent with beneficial effects against AS [27,35,36].
Melatonin and atherosclerosis
Atherosclerosis is a chronic inflammation that is amplified by the effect of oxidative stress. Melatonin has shown to have an athero-protective role in chronic inflammation by reducing pro-inflammatory mediators and transcription factors, such as the inhibiting the interaction between NF-κB and TLR-4 [30] and NLRP3 [37]. As mentioned, oxidative stress plays a key role in AS plaque progression. Melatonin, through its antioxidant effect, may have the ability to reduce AS progression. In addition to melatonin’s anti-inflammatory and anti-oxidant mechanisms, various studies have shown melatonin to inhibit AS plaque progression through other pathways [38,39]. Zhang et al. showed that melatonin enhances atherosclerotic plaque stability by inducing prolyl-4-hydroxylase α1 (P4HA1) expression, which is necessary for collagen maturation [40]. Cheng et al. exhibited melatonin to have an athero-protective effect through regulating Mitogen-Activated Protein Kinase (MAPK) pathway in a rabbit atherosclerotic model [41].
In conclusion, considering the severe complications and high mortality of atheroclerosis accompanied by insufficient efficacy of current anti-atherosclerotic treatments, there is a strong need for developing novel strategies for AS treatment.
Hypothesis
Atherosclerosis is the main cause of the CVD leading cause of mortality in the world. Recent studies have attempted to develop novel anti-atherosclerotic treatments. It has been demonstrated that AS is a chronic inflammation of large and medium vessels. Studies show that oxidative stress and inflammation play a key role in AS plaque progression. Thus, anti-inflammatory and anti-oxidant agents have shown to represent promising effects in the regression of AS plaques. Also, T reggs have anti-inflammatory activities due to their immune suppressing activity that can end in AS plaque regression.
In this article, we introduce melatonin as an athero-protective agent due to its antioxidant activity through free radical scavenging and its anti-inflammatory role in chronic inflammation by reducing the expression of pro-inflammatory genes. Some studies have reported exogenous administration of melatonin to have promising results in AS plaque regression.
According to the advantages of the gene therapy in atherosclerosis, it could be considered as an alternative strategy against AS. The over expression of some molecules or substances that have athero-protective effects can lead to promising results in the treatment of atherosclerosis. Considering the athero-protective effects of melatonin, it could be introduced as a new candidate for gene therapy of AS.
As mentioned before, studies have shown the benefits of exogenous administration of melatonin in AS plaque; however, none of them have examined the endogenous effect of melatonin. For the first time, in order to evaluate the endogenous production of melatonin and its effect on AS plaque, we hypothesize the over expression of the melatonin rate-limiting enzymes (AANAT and HIOMT) genes in T regs in mice model of atherosclerosis. This approach could be proposed as a novel gene therapy approach to inhibit the progression of AS plaque. Also,the athero-protective properties of T regs could facilitate the atheroprotective effects of endogenous melatonin production by its immune-suppressing effects.
Evaluation of hypothesis
For testing hypothesize we recommend the following experiments (figure 1):
1. Animal: C57BL/6 mice that have deficient Apo-lipoprotein E (ApoE -/-) are a suitable model for studying AS. A total of 30 male, 8 weeks old mice are going to be prepared. Our intervention involves the injection of modified T regs that produce melatonin. Mice are going to be randomly divided into three groups (n=10 in each groups):
a. First group will be fed with western type diet (which includes 15% fat and 21% fat that is originated by milk) and no intervention for 12 weeks (AS group).
b. Second group mice will be fed by western type diet for 12 weeks, and melatonin intervention will be started 4 weeks after feeding (case group).
c. Third group will be fed by western type diet for 12 weeks and empty vector will be injected 4 weeks after feeding (control group).
d. According to the immune suppressor activity of the T reggs, the mice will be kept in pathogene free status.
2. T cell isolation: T cells will be isolated from mice peripheral blood through the routine techniquesusing Ficoll-hypack.
3. Cell culture media: T cells will be cultured with RPMI-1640 with 10% fetal bovine serum (heat-inactivated), 1.5 mg/mL sodium bicarbonate, 10 mM HEPES, 1mMsodium pyruvate, and 100 U/mL penicillin and streptomycin. The complete medium should be stored at 4 oC after preparation and pre-warmed to 37 oC before use.
4. T reggs production: In order to obtain T regs, CD4+ T cells will be induced to express FOXP3 (T reggs specific marker) by adding IL-2 and TGF-β1 to the culture media.
5. T reggs production evaluation: T reggs are recognized with CD4, CD25, and FOXP3 markers. Anti-FOXP3 antibodeis will be used with ELISA kit to identify and isolate T reggs.
6. Construction of the vector for AANAT gene (pIRES2-EGFP-AANAT): In order to obtain total AANAT gene RNA, pineal gland is going to be utilized according to the high expression of AANAT gene in this organ. After cloning the gene, it will be extracted and cloned into pIRES2-EGFP plasmid vector for transfection.
7. Prepared modified T cells: After preparing the pIRES2-EGFP-AANAT vectors, T regs will be induced for division by phytohemaglutinin (PHA). Then, the vectors are going to be transfected to the T regs through lipofectamin method.
8. Melatonin production assay: To evaluate the melatonin level in 4 groups of mice, blood will be collected from caudal vein and melatonin serum level will be assayed by radioimmuno assay.
9. Inflammatory factors evaluation: To evaluate the anti-inflammatory effect of melatonin,the serum level of pro-inflammatory factors including IL-1, 6 and TNF-αwill be measured by ELISA during the cure period.
10. AS plaque assay during 12 weeks: It has been deomonstrated that Cluster of Differentiation 81 protein (CD81) is upregulated in endothelium of AS plaque. Thus, all of the mice are going to be imaged by molecular Magnetic Resounance Imaging (MRI) usnig CD81 anitbody-conjucated Micron-sized Particles of Iron Oxide [42].
11. After 8 weeks of T cell infusion, all mice undergo euthanasia and will be sacrificed by cervical dislocation. To examine the AS plaque progression and MLT effect, carotid and descending aorta will be removed. Samples will be stainedusinghaemotoxylin and eosin (H&E) staining.
12. AS plaque stability: Since melatonin has effect on plaque stability, plaques will be stained by Picrosirius Red, to appraise collagen level in plaques.
Conclusion & discussion
Atherosclerosis (AS) is a chronic inflammation of the large and medium vessels. Briefly, it is initiated by accumulation of LDL. Further immune response promtes the progression of AS plaques. Since there is no definite cure for AS and its severe complications, such as MI and stroke, further research is essential for reaching highly effective treatments. Gene therapy has recently gained more attention in the treatment of AS [43,44] and multiple other diseases [45-50].
In this hypothesize, we introduce melatonin as an athero-protective molecule. Its athero-protective effect are exerted through both anti-inflammatory and anti-oxidative properties. MLT is an anti-oxidant due to its free radical scavenging activity. Also, its anti-inflammatory activity has been demonstrated in chronic inflammation through down regulation of pro-inflammatory genes. MLT can also inhibit the activation of NLRP3 inflammasome [37] and reduce TLR-4 expression(30), which are responsible for inflammatory cytokine secretion. In another way, MLT can stabilize AS plaque by inducing P4HA1 [40], the enzyme responsible for collagen maturation. The athero-protective activities of melatonin are not limited to these pathways and studies have shown other mechanisms, as well.
Regarding promising reduction in AS plaque after administration of exogemnous melatonin, we hypothesize that MLT could be a good candidate for gene therapy of AS. To reach this goal, we hypothesize the overexpression of AANAT (rate limiting enzyme responsible for MLT biosynthesize) gene in T reggs using pIRES2-EGFP-AANAT vector.
Based on the anti-inflammatory, anti-oxidative, and plaque-stabilizative effects of melatonin, we expect the endogenous overexpression of melatonin by T regs to:
a) Overexpress AANAT gene and increase serum level of MLT in treated group versus other groups.
b) Decrease inflammatory mediators, such as IL-1B, 2,18 and, MCP-1 in T reg treated group versus control group
c) Reduce in AS plaque diameter using H&E staining in treated group versus control group
d) Increase in the stability of AS plaque due to higher collagen level in treated group compared to control group.
Briefly, based on the facts that were reviewed, we expect the endogenous melatonin overexpression to exhibit athero-protective effects and to have promising regression in AS. Also, the endogenous production of melatonin by T regs is expected to show less side effects than the exogenous infusion, because of the endogenous regulation of melatonin production by T regs. However, further studies will be required to evaluate our expectations.
Various studies have shown the effects of exogenous melatonin administration in AS. Sai et al. in 2018 reported the intraperitoneal administration of MLT to significantly reduce AS progression through inhibition of NLRP3 inflammasome in ApoE-/- mice [37]. Ze-Ping et al. examined the administration of MLT via gavage in male New Zealand white rabbits which showed acceptable regression of AS plaque. This effect was administered through suppression of TLR4/NF-kβ system that resulted in reduction of inflammatory mediators secretion [30]. Hongxuan Li et al. showed MLT to increase AS plaque stability by inducing P4HA1 expression that is necessary for collagen maturation [40].
Similar to our hypothesize, Jingli et al. in 2018 showed the effect of AANAT overexpression on the inflammation in transgenic goats. For the first time, they revealed the effect of endogenous melatonin production in inflammation. They demonstrated that the in-vitro production of MLT to the PBMCs, accompanied by Lipopolysacharide (LPS) challenge, led to an anti-inflammatory effect through induction of cellular autophagy in PBMCs. Despite reduce inflammation in in-vitro enviroenment, the in-vivo endogenous production of melatonin by PBMCs, accompanied by LPS challenge, led to increased inflammation [51]. The controversy in this study can be explained by differences in-vitro and in-vivo environments, the overexpression of MLT in multiple cellular lineages, and acute inflammation induced by LPS injection.
Recent studies have demonstrated promising athero-protective activities of of MLT on AS plaque [39,40]. So we hypothesized that endogenous MLT production may have athero-protective activity, as well. To evaluate this hypothesis and due to advantages of gene therapy, the authors propose AANAT gene overexpression in T regs using gene therapy, could end in endogenous melatonin production in APO E-/- mice. Production of melatonin by T regscould consequently reduce AS plaque progression and size.
Although melatonin is physiologically produced in the body, high concentrations of melatonin in the blood could result in some side effects. The main side effects consist of headache, dizziness, nausea, and drowsiness. The rare side effects that must also be considered are interaction with some medications, including anticoagulants, anti-platelet drugs, contraceptive drugs, diabetes medications, and medications that suppress the immune system. These are the most common side effects of melatonin [52] (https://www.webmd.com/vitamins/ai/ingredientmono-940/melatonin).
Also, due to immune suppressive activity of T reggs, we suggest that mice should be kept in pathogene free condition. Considering personalized medicine, since the gene polymorphism in drug-metabolizer enzymes affect the metabolism of melatonin in the liver, patients with CYP1A2 to 6-hydroxymelatonin polymorphism in cytochrome P450 enzyme are suggested not to be prescribed melatonin [53].
References
- Moss JW, Ramji DP. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future medicinal chemistry. 2016; 8: 1317-1130.
- Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine & growth factor reviews. 2015; 26: 673-685.
- Moriya J. Critical roles of inflammation in atherosclerosis. Journal of cardiology. 2019; 73: 22-27
- Fatkhullina A, Peshkova I, Koltsova E. The role of cytokines in the development of atherosclerosis. Biochemistry (Moscow). 2016; 81: 1358-1370.
- Ensan S, Li A, Besla R, Degousee N, Cosme J, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nature immunology. 2016; 17: 159.
- Wang X, Sun Y, Yang H, Lu Y, Li L. Oxidized low-density lipoprotein induces apoptosis in cultured neonatal rat cardiomyocytes by modulating the TLR4/NF-κB pathway. Scientific reports. 2016; 6: 27866.
- Buckley ML, Ramji DP. The influence of dysfunctional signaling and lipid homeostasis in mediating the inflammatory responses during atherosclerosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015; 1852: 1498-1510.
- Abdolmaleki F, Hayat SMG, Bianconi V, Johnston TP, Sahebkar A. Atherosclerosis and immunity: A perspective. Trends in cardiovascular medicine. 2019; 29: 363-371.
- Nguyen MT, Fernando S, Schwarz N, Tan J, Bursill CA, et al. Inflammation as a therapeutic target in atherosclerosis. Journal of clinical medicine. 2019; 8: 1109.
- Ilhan F, Kalkanli ST. Atherosclerosis and the role of immune cells. World Journal of Clinical Cases: WJCC. 2015; 3: 345.
- Rhoads JP, Major AS. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Critical Reviews™ in Immunology. 2018; 38.
- Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. International journal of endocrinology. 2017; 2017.
- Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, et al. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Frontiers in physiology. 2018; 9: 114.
- Wiesner P, Tafelmeier M, Chittka D, Choi S-H, Zhang L, et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein (a) in human plasma. Journal of lipid research. 2013; 54: 1877-1883.
- Cherubini A, Vigna GB, Zuliani G, Ruggiero C, Senin U, et al. Role of anti-oxidants in atherosclerosis: Epidemiological and clinical update. Current pharmaceutical design. 2005; 11: 2017-2032
- Jovinge S, Ares MP, Kallin B, Nilsson J. Human monocytes/macrophages release TNF-α in response to ox-LDL. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996; 16: 1573-1579.
- Wang Y-C, Hu Y-W, Sha Y-H, Gao J-J, Ma X, et al. Ox-LDL upregulates IL-6 expression by enhancing NF-κB in an IGF2-dependent manner in THP-1 macrophages. Inflammation. 2015; 38: 2116-2123.
- Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, et al. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. Journal of Biological Chemistry. 1996; 271: 8837-8842.
- Wintergerst ES, Jelk J, Rahner C, Asmis R. Apoptosis induced by oxidized low density lipoprotein in human monocyte‐derived macrophages involves CD36 and activation of caspase‐ European Journal of Biochemistry. 2000; 267: 6050-6059.
- Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011; 216: 313-320.
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, et al. Atherosclerosis. Nat Rev Dis Primers. 2019; 5: 56.
- Riccioni G, Sblendorio V. Atherosclerosis: from biology to pharmacological treatment. Journal of geriatric cardiology: JGC. 2012; 9: 305.
- Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nature reviews Drug discovery. 2011; 10: 365-376
- Rader D. Gene therapy for atherosclerosis. International Journal of Clinical and Laboratory Research. 1997; 27: 35-43.
- Kassim SH, Li H, Bell P, Somanathan S, Lagor W, et al. Adeno-associated virus serotype 8 gene therapy leads to significant lowering of plasma cholesterol levels in humanized mouse models of homozygous and heterozygous familial hypercholesterolemia. Human gene therapy. 2012; 24: 19-26.
- Liu Y, Li D, Chen J, Xie J, Bandyopadhyay S, Zhang D, et al. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006; 188: 19-27.
- Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochemical pharmacology. 2010; 80: 1844-1852.
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Molecular and cellular endocrinology. 2012; 351: 152-166.
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of pineal research. 2007; 42: 28-42.
- Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, et al. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR 4/NF‐κB system in high‐fat‐fed rabbits. Journal of pineal research. 2013; 55: 388-398.
- Li H-Y, Leu Y-L, Wu Y-C, Wang S-H. Melatonin inhibits in vitro smooth muscle cell inflammation and proliferation and atherosclerosis in apolipoprotein e-deficient mice. Journal of agricultural and food chemistry. 2019; 67: 1889-1901.
- Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan D-X, et al. Role of melatonin in the regulation of autophagy and mitophagy: a review. Molecular and cellular endocrinology. 2012; 361: 12-23.
- Sun Y, Guan X-r. Autophagy: A new target for the treatment of atherosclerosis. Frontiers in Laboratory Medicine. 2018; 2: 68-71.
- Boga JA, Caballero B, Potes Y, Perez‐Martinez Z, Reiter RJ, et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. Journal of pineal research. 2019; 66: e12534.
- Reiter RJ, Tan D-x, Mayo JC, Sainz RM, Leon J, et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. ACTA BIOCHIMICA POLONICA-ENGLISH EDITION-. 2003; 50: 1129-1146.
- Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, et al. Regulation of antioxidant enzymes: A significant role for melatonin. Journal of pineal research. 2004; 36: 1-9.
- Ma S, Chen J, Feng J, Zhang R, Fan M, et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxidative medicine and cellular longevity. 2018; 2018
- Favero G, Rodella LF, Reiter RJ, Rezzani R. Melatonin and its atheroprotective effects: A review. Molecular and cellular endocrinology. 2014; 382: 926-937.
- Sun H, Gusdon AM, Qu S. Effects of melatonin on cardiovascular diseases: progress in the past year. Current opinion in lipidology. 2016; 27: 408.
- Li H, Li J, Jiang X, Liu S, Liu Y, et al. Melatonin enhances atherosclerotic plaque stability by inducing prolyl-4-hydroxylase α1 expression. Journal of hypertension. 2019; 37: 964-971.
- Tang S-t, Su H, Zhang Q, Tang H-q, Wang C-j, et al. Melatonin attenuates aortic endothelial permeability and arteriosclerosis in streptozotocin-induced diabetic rats: Possible role of MLCK-and MLCP-dependent MLC phosphorylation. Journal of cardiovascular pharmacology and therapeutics. 2016; 21: 82-92.
- Yan F, Yang W, Li X, Liu H, Nan X, et al. Magnetic resonance imaging of atherosclerosis using CD81-targeted microparticles of iron oxide in mice. BioMed research international. 2015; 2015.
- Ahmadi F, Esmaeilzadeh A. IL-1R2: a novel approach for gene therapy in atherosclerosis. Hypothesis J. 2016; 14: e1.
- Esmaeilzadeh A, Pouyan S, Erfanmanesh M. Is Interleukin-38 a key player cytokine in atherosclerosis immune gene therapy? Medical hypotheses. 2019; 125: 139-43.
- Khosh E, Bahmaie N, Esmaeilzadeh A. Evolution in Immune Gene Therapy of Glioblastoma; Interleukin-37 as a Novel Candidate. Clin Oncol. 2019; 4: 1618.
- Manesh ME, Esmaeilzadeh A, Mirzaei MH. IL-24: A novel gene therapy candidate for immune system upregulation in Hodgkin’s lymphoma. Journal of Medical Hypotheses and Ideas. 2015; 9: 61-66.
- Mirzaei MH, Esmaeilzadeh A. Overexpression of MDA-7/IL-24 as an anticancer cytokine in gene therapy of thyroid carcinoma. Journal of Medical Hypotheses and Ideas. 2014; 8: 7-13.
- Mirzamohammadi F, Esmaeilzadeh A. A Novel Method in Gene Therapy of HIV. Iranian Journal of Public Health. 2007; 36.
- Moghadam S, Erfanmanesh M, Esmaeilzadeh A. Interleukin 35 and Hepatocyte Growth Factor; as a novel combined immune gene therapy for Multiple Sclerosis disease. Medical hypotheses. 2017; 109: 102-105.
- Piri Z, Esmaeilzadeh A, Hajikhanmirzaei M. Interleukin-25 as a candidate gene in immunogene therapy of pancreatic cancer. Journal of Medical Hypotheses and Ideas. 2012; 6:75-79.
- Tao J, Yang M, Wu H, Ma T, He C, et al. Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy. 2018; 14: 1850-1869.
- Hacışevki A, Baba B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. Melatonin-Molecular Biology, Clinical and Pharmaceutical Approaches: IntechOpen; 2018.
- Skene DJ, Papagiannidou E, Hashemi E, Snelling J, Lewis DF, Fernandez M, et al. Contribution of CYP1A2 in the hepatic metabolism of melatonin: Studies with isolated microsomal preparations and liver slices. Journal of pineal research. 2001; 31: 333-342.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.