Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Myocardial Injury, Inflammation and Prothrombotic Response Are Associated with Outcomes of COVID-19 Patients
- Rachel T Scarl;
- Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.
- Joan-Miquel Balada-LIasat;
- Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.
- Nicholas Nowacki;
- Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.
- R John Solaro;
- Department Physiology and Biophysics, Center for Cardiovascular Research, College of Medicine, University of Illinois at Chicago, Chicago, Illinois, USA.
- JoAnna Williams*;
- Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.
- Jieli Li*
- Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.

| Received | : | Dec 24, 2020 |
| Accepted | : | Feb 02, 2021 |
| Published Online | : | Feb 05, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Scarl RT, Balada-LIasat JM, Nowacki N, Solaro RJ, Williams J, et al. Myocardial Injury, Inflammation and Prothrombotic Response Are Associated with Outcomes of COVID-19 Patients. Ann Cardiol Vasc Med. 2021: 4(1); 1041.
Abstract
Background: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which causes Coronavirus Disease 2019 (COVID-19) has rapidly grown into a pandemic. SARS-CoV-2 is known to affect the multi-organ systems including myocardial injury. Acute cardiac injury determined by elevated serum troponin levels has been observed in severe cases and is strongly associated with mortality. This study is a retrospective review to investigate the association between myocardial injury, systemic inflammation and prothrombotic response in COVID-19 patients.
Methods: This retrospective study analyzed hospitalized patients with COVID-19 at the Ohio State University Wexner Medical Center, in March-April 2020. A total of 81 hospitalized patients were included in the study. Demographic data, medical history, comorbidities, and laboratory findings were collected and analyzed.
Results: The survival rate was significantly associated with pre-existing Cardiovascular Diseases (CVD), hyperlipidemia, and Acute Respiratory Distress Syndrome (ARDS). Dynamic elevation of cardiac troponin I (cTnI), CRP, Interleukin 6 (IL-6), ferritin, Procalcitonin (PCT) and D-Dimer were found to be associated with the prognosis of patients. The peak levels of cTnI, CRP, IL-6, ferritin, PCT and D-Dimer was significantly elevated, while lymphocytes counts were dramatically reduced in non-survival patients during hospitalization (p<0.05).
Conclusions: COVID-19 should be considered as an inflammatory disease. The pro-inflammatory cytokines or acute phase proteins induced by SARS-CoV-2 might initiate a cascade of signaling pathways which leads to cardiomyocyte apoptosis and troponin releasing.
Introduction
Coronavirus Disease 2019 (COVID-19), a novel disease caused by coronavirus SARS-CoV-2, has sickened millions and continues to affect large numbers of people worldwide. Cardiac Troponins (cTns) are sensitive biomarkers of acute myocardial infarction, and nonischemic myocardial injury [1]. Myocardial injury is evidenced by elevated serum troponins above the 99th percentile upper reference limit, according to the Fourth Universal Definition of Myocardial Infarction [2]. Myocardial injury is common in COVID-19 patients, presenting with increase of cardiac troponins, especially in patients with underlying Cardiovascular Diseases (CVDs) who have an increased risk of death [3-8]. Patients with comorbidities such as obesity, hyperlipidemia or diabetes are more likely to have severe disease and higher mortality [9].
Cytokine Release Syndrome (CRS) is a severe immune reaction in which the body releases substantial amounts of cytokines such as Interferon (INF)-γ, Interleukin (IL)-1 and IL-6 in a short amount of time. Although cytokines play a vital role in the normal immune response, extensive release can be life-threatening, leading to multiple organ failure. Preliminary studies have shown increased levels of these cytokines in patients for at least two weeks following the onset of COVID-19 symptoms suggesting their role in poor patient outcomes [4,10].
Inflammatory markers are a useful way to measure the progression and response to treatment of infectious and/or inflammatory processes. C-Reactive Protein (CRP) is an acute-phase protein produced by the liver during bacterial infections and inflammation. Ferritin is another pro-inflammatory cytokine, correlating with inflammatory activity. It has been postulated that cytokines and ongoing inflammation might be associated with COVID-19 disease severity. In a retrospective study, with 150 confirmed COVID-19 patients, ferritin and CRP were dramatically elevated in non-survivals [10], suggesting the high mortality might be due to virally driven hyper-inflammation. D-Dimer concentrations were much higher in patients with critical COVID-19 [11], suggesting the significant associations between D-Dimer and severity in COVID-19. Elevated Procalcitonin (PCT) levels not only indicate bacterial infection but also the systemic inflammatory reaction [12,13]. In patients with COVID-19, PCT, IL-6 and CRP are elevated very early in blood and remains in a high level [14]. It has been also reported that PCT, IL-6 and CRP are released in Acute Myocardial Infarction (AMI) as a response to the ensuing inflammatory process or/and to the tissue injury [15,16]. Therefore, myocardial injury in COVID-19 patients might be caused by inflammation, which needs to be investigated.
The severity of COVID-19 may be hidden by an initial mild presentation of SARS-CoV-2 infection, with less patients experiencing fever, chills, chest tightness and shortness of breath. This may cause a life-threatening delay in providing the needed care, resulting in a poorer prognosis. Therefore, underestimation of COVID-19 severity in patients needs further attention. In this retrospective study, we aimed to understand the connection between myocardial injury, inflammation, prothrombotic response and the prognosis in COVID-19 patients. We have looked at the effects of cardiac injury and cytokine levels on overall patient mortality. A total of 81 hospitalized COVID-19 patients at the Ohio State University Wexner Medical Center were included in the retrospective study. By understanding the damage caused by SARS-CoV-2 to the cardiovascular system and the underlying mechanisms of injury, effective treatment of these patients can be given in an appropriate and timely manner while reducing overall mortality.
Methods
Clinical subjects
This is a single-center, retrospective, observational study conducted at the Ohio State University Wexner Medical Center, approved by the Institutional Review Board at the Ohio State University. A total of 81 hospitalized COVID-19 patients from Mar-Apr 2020, who were either treated and discharged or died during hospitalization, were included. COVID-19 was confirmed by molecular testing from of nasopharyngeal specimens. Only laboratory confirmed cases were included in the study. The electronic medical records of these patients were reviewed, and patient data including demographics, diagnosis, medical history, and laboratory results were collected. Laboratory studies included serum Cardiac Troponin I (cTnI), CRP, PCT, ferritin, D-Dimer, lymphocytes counts and IL-6.
Statistical analysis
Ages were expressed as medium and interquartile ranges. Categorical variables were summarized as counts and percentages. Comparisons between two groups were performed by Chi square test. One-way ANOVA was performed by SPSS 22. A p<0.05 was considered statistically significant.
Results
Data, summarized in Table 1, were collected on hospitalized patients with COVID-19, including 64 patients who were successfully treated and discharged and 17 patients who died. Six discharged patients were excluded because of incomplete data of serum cTnI and cytokines. As shown in Table.1, patients in non-survival group were older than the survival group [69 years (IQR, 42-93) vs. 56 years (IQR, 23-87)]. Of the 81 patients, 48 (60%) had pre-existing CVDs including Coronary Heart Disease (CAD), Myocardial Infarction (MI), cardiac hypertrophy, arrhythmia, Chronic Heart Failure (CHF) and 33 (40%) did not. The 81 patients were divided into two groups according to the serum level of cTnI, the specific biomarker of myocardial injury, cTnI negative group (cTnI ≤0.04 ng/mL, n=43) and cTnI positive group (>0.04 ng/mL, n=20). Of the cTnI positive patients, 50% were non-survival, whereas 5% in cTnI negative group (p<0.0001) (Table 1). Data in (Figure 1A), show that the cTnI levels in the non-survivals were dynamically elevated, compared with the survival group. Of the 20 patients with elevated cTnI, 75% had pre-existing CVDs, whereas 51% in patients with negative cTnI results (p= 0.1). Of the survival patients, 53% had pre-existing CVDs, and in non-survival group, 83% had (p= 0.05) (Table 1).
With evaluation of cardiac injury risks, 44% of total 81 patients had HTN. Of the positive cTnI patients, 60% had HTN, while 37% had HTN in the group with negative cTnI. Similarly, in the survival group, 45% had HTN while 47% in the group of non-survival had HTN, and there is no statistic significances noticed between these two groups (Table 1). Of the total patients, 33% had Hyperlipidemia (HLD). However, 65% of cTnI positive had hyperlipidemia, while only 28% of cTnI negative patients had hyperlipidemia. This difference was statistically significance (p= 0.007). The statistical significance of hyperlipidemia was also observed in survival and non-survival groups. Of the patients who survived, 19% had hyperlipidemia, whereas in those who died, 88% had hyperlipidemia (p<0.0001) (Table 1).
We further investigated the comorbidities in patients. Our findings showed no statistical significance in CAD, cardiac hypertrophy, arrhythmia, MI, CHF, obesity, and pneumonia caused by COVID-19 in either cTnI positive or negative groups, suggesting these comorbidities might not be risk factors for cardiac injury in COVID-19 patients. As described above, ARDS is one of the leading causes of mortality in COVID-19 patients. Of cTnI positive patients, 55% had ARDS, compared with 21% of cTnI negative group (p= 0.009), indicating that ARDS might be a potential cause of cardiac injury. Moreover, in these patients, 65% of the non-survival group vs. 27% of the survival group had ARDS (p= 0.008).
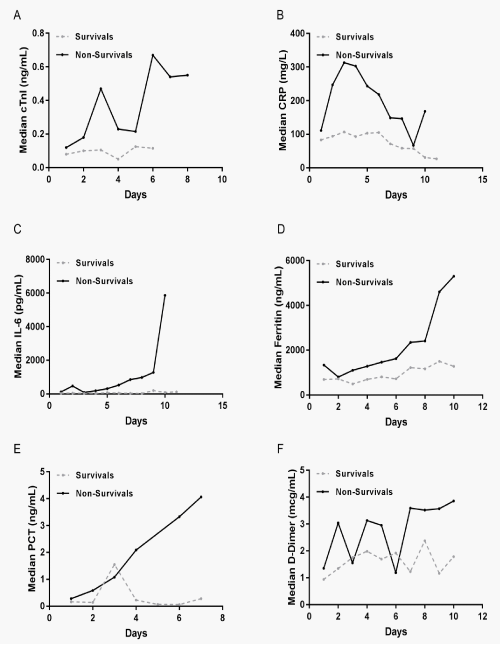
To investigate the role of inflammation and prothrombotic response in COVID-19 patients, patients were divided into survival and non-survival groups. The medians for CRP, IL-6, ferritin, PCT and D-Dimer were displayed separately (Figure 1B-F). The graph illustrates the large variations of the different biomarkers and the patterns of change within each group. In the non-survival group, the changes of CRP, IL-6, ferritin, PCT and D-Dimer were notably elevated (Figure 1B-F).
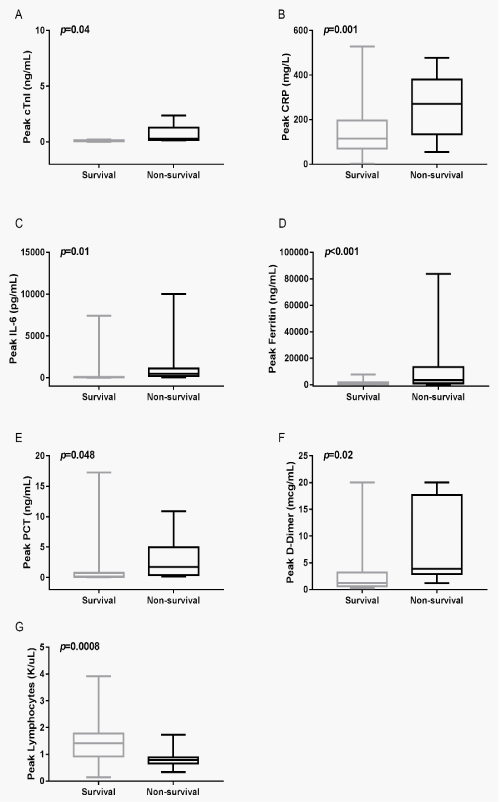
Demonstrates the changes of cTnI and CRP, IL-6, ferritin, PCT, D-Dimer, lymphocytes counts at the peak levels during hospitalization in the survival and non-survival group (Figure 2). Comparison analysis of peak cTnI, CRP, IL-6, ferritin, PCT and D-Dimer in the survival and non-survival patients showed significantly elevation in the non-survival patients (Figure 2A-F). However, the lymphocytes counts showed dramatically reduction in the non-survival patients (p=0.0008) (Figure 2G).
Figure 1: Patterns of variations described by medians overt time for the outcome cTnI, D-Dimer and biomarkers of inflammation in COVID-19 patients. The daily median values of cTnI, CRP, IL-6, Ferritin, PCT and D-Dimer in their original units were graphically displayed to illustrate the variations of each biomarker over time in the groups of survivals and non-survivals.
Figure 3: Peak levels of cTnI, the acute-phase proteins CRP, ferritin, PCT and cytokines IL-6 in the survival and non-survival patients during the hospitalization.
(A) Peak levels of cTnI in the survival and non-survival patients,
(B) Peak levels of CRP in the survival and non-survival patients,
(C) Peak levels of IL-6 in the survival and non-survival patients,
(D) Peak levels of ferritin in the survival and non-survival patients,
(E) Peak levels of PCT in the survival and non-survival patients,
(F) Peak levels of D-Dimer in the survival and non-survival patients,
(G) Peak levels of lymphocytes counts in the survival and non-survival patients.
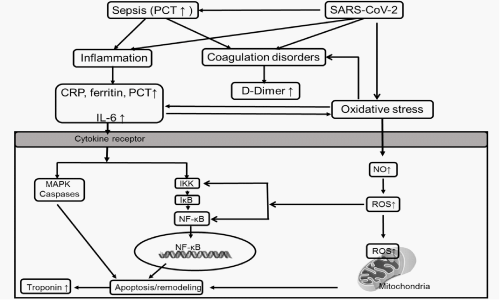
Figure 3: Hypothesis of the potential mechanism contributing to cardiomyocyte apoptosis/remodeling in COVID-19 patients. The cytokines such as IL-6 affected striated muscle mass through activation of mitogen-activated protein kinase (MAPK) caspases or activation of the IκB Kinases (IKK) to promote the phosphorylation and subsequent degradation of IκB, enabling nuclear translocation of the transcription factor Nuclear Factor (NF)-κB and the subsequent expression of genes involved in protein degradation to induce the apoptosis of cardiomyocytes. PCT plays an important role in either sepsis inducing or inflammatory mediators in the signaling pathways in cardiomyocytes apoptosis. The cytokines are known potent stimulators of the oxidative stress. And there is the crosstalk between cytokines and oxidative stress. Oxidative stress also could be induced directly by SARS-CoV-2. The Reactive Oxygen Species (ROS) induced in oxidative stress leads to mitochondrial dysfunction in cardiomyocytes, resulting in myocardial apoptosis. The oxidative stress in COVID-19 patients triggers venous thrombosis and thereby D-Dimer increased.
Discussion
Our retrospective and observational study of adults, who were hospitalized with COVID-19 at the Ohio State University Wexner Medical Center, and assessed with the biomarkers to identify the risk factors for mortality that are important for patient management. The mortality in COVID-19 patients with underlying CVD reached 10.5% in a previous study evaluating patients in China [17], whereas it was markedly higher (29%) in COVID-19 patients with CVD in the current study (data not shown). Our study also demonstrates that patients with severe disease leading to fatality had elevated serum cTnI levels compared to those who survived. cTnI, CRP, IL-6, ferritin, PCT and D-Dimer were all dynamically elevated in the non-survival patients. In addition, peak levels of cTnI, CRP, IL-6, ferritin, PCT and D-Dimer were all elevated during the hospitalization, indicating the association of myocardial injury and systemic inflammation and hypercoagulability in COVID-19 patients. Strong association between ARDS and COVID-19 patients was identified in the study. In addition, we found 55% of COVID-19 patients with myocardial injury had ARDS, while 21% of COVID-19 patients without myocardial injury had ARDS (p=0.009). Hypoxia due to ARDS likely results in myocardial injury through cardiac myocyte injury [18], leading to relatively high mortality. Hypoxia/ischemia and demand ischemia are well known to result in loss of Adenosine Triphosphate (ATP), cell swelling, bleb formation and rupture, which were identified as the first indications that serum cTn, which is uniquely expressed in cardiac myocytes, could be employed as a biomarker for Acute Myocardial Infarction (AMI) [19,20]. It has been reported that an increase in serum cTn may occur with induction of membrane fragility associated with adverse “inside-out” mechanical signaling via cytoskeletal proteins in the face of ischemic demand [1]. These are the likely mechanism by which myocytes release cTn levels in COVID-19 patients in which there is evidence of micro-circulatory changes inducing hypoxia [21,22].
A role for hyperlipidemia, which was a significant co-morbidity and associated with elevated serum cTnI in the present study, is well established in the literature to be associated with cardiac myocyte cell death in diabetic patients [23]. Animal models of diabetes and high fat induced obesity commonly demonstrate pathologies leading to release of cTn into serum including elevated Reactive Oxygen Species (ROS), inflammation with IL-6 and Tumor Necrosis Factor Alpha (TNF-α), and inefficient generation of ATP resulting in ROS and reduced contractility, ischemia and increased demand ischemia [24,25]. The role of hyperlipidemia in cell death has also been supported by studies showing that the pathologies mentioned above and elevated serum cTn are reversed by long term treatment of diabetic rats with simvastatin [24]. Reversal of the changes induced in serum cTn and pathologies also occurred in diabetic models treated with an AT1R blocker (Azilsartan) [26] and bioactive flavonol, fisetin [27]. A study of 195 patients undergoing percutaneous coronary intervention provided data in which use of high sensitivity cTn antibodies predicted severe myocardial injury associated with elevated serum lipids [28]. These data, which indicated a need for aggressive treatment of the hyperlipidemia prior to the percutaneous coronary intervention, suggest the same decision possibly needed in COVID-19 patients. It is also relevant to the present study that the decision on whether to use a statin can be made on the basis of determination of levels of serum cTn.
CAD has been reported to be associated with acute cardiac events and poor outcomes in influenza or other respiratory viral infections [29-30], however, in the current study, the mortality in CAD patients was only 24%. In the current study, in patients with myocardial injury, 20% had CAD, suggesting its possible minimal role in myocardial damage in patients with COVID-19.
Some viruses can trigger the intracellular cascade and the release of IL-6, which contributes to the inflammatory status. An example of this is SARS-Cov-1 which significantly induced the activation of IL-6 promoter in human airway epithelial cell cultures [31]. IL-6 has been paid attention in this COVID-19 pandemic [32]. Early in the outbreak, IL-6 was reported as a reliable indicator of requiring ICU admission [33], with 52% increase in the level of IL-6 in ICU patients compared to non-ICU [34]. In addition, IL-6 also has significant association with disease severity and works as a predictor in the ventilation support [35]. Mechanisms for cTn elevations in serum also include the cytokine release storm in SARS-CoV-2 patients [36]. A cogent example is the rise of IL-6 in disorders such as AMI and CAD [16], which are conditions well known to correlate with elevations in serum cTn. The in vitro or animal studies also provided evidence that cytokines such as IL-6 affected striated muscle mass through activation of Mitogen-Activated Protein Kinase (MAPK) caspases [37, 38] or activation of the IκB Kinases (IKK) to promote the phosphorylation and subsequent degradation of IκB, enabling nuclear translocation of the transcription factor Nuclear Factor (NF)-κB [39] and the subsequent expression of genes involved in protein degradation to induce the apoptosis of cardiomyocytes. Here comes our hypothesis which is cytokines/inflammation biomarkers released by SARS-CoV-2 might trigger the caspases of signaling pathways to induce cardiomyocyte apoptosis/remodeling and release troponin into the circulation (Figure 3). In the current study, we investigated the association of elevated cytokine IL-6, as well as systemic inflammatory responses, had a synergistic effect in myocardial injury. The association between IL-6 and cTnI were observed in non-survival patients in the current study, indicating IL-6 might play an important role in myocardial injury. However, interventions targeting single IL-6 to treat patients with COVID-19 [40], unfortunately, reported failures [41], which indicates there might be other cytokines or inflammation factors working together resulting in the myocardial injury in COVID-19 patients rather than IL-6 alone.
Similarly, the elevations of several acute-phase biomarkers were found in patients with COVID-19. High levels of CRP, and ferritin [42] have been observed in patients with severe diseases. In our study, in the non-survival patients, peak levels of cTnI, CRP and ferritin were all significantly elevated in non-survival patients, suggesting the roles of inflammatory in resulting in myocardial injury. These cytokines/inflammation mediators are known potent stimulators of oxidative stress in heart failure, obesity or diabetes [43]. However, we believe there is the crosstalk between cytokines and oxidative stress in COVID-19 patients. Several in vitro and in vivo studies have highlighted the initiation of oxidative stress by virus infection, which plays an important role in the activation of innate immunity by generating cytokines. For example, Reactive Oxygen Species (ROS) production was induced by Respiratory Syncytial Virus (RSV) infection, which induced the expression of pro-inflammatory cytokines [44-46]. In addition, oxidative stress also could be induced directly by virus for their replication inside the cell [47]. Therefore, oxidative stress might be importantly involved in the SARS-CoV-2 infection. High levels of ROS production in cardiomyocytes might overwhelm cellular antioxidant defense systems, resulting in adverse cardiac remodeling. Myocardial oxidative stress impairs cardiac function probably by oxidative damage to cellular proteins, thereby inducing cellular apoptosis. Thus, oxidative stress plays a role in the myocardial injury of COVID-19, through either direct tissue injury with ROS production and mitochondrial dysfunction in cardiomyocytes or by the crosstalk with the cytokines production. It is reasonable to propose a pathogenesis model of myocardial injury caused by SARS-CoV-2, which is supported by robust experimental evidence presented by the scientific literature (Figure 3).
PCT is a diagnostic biomarker for sepsis and antibiotic therapy [48]. The pathophysiology of sepsis includes inflammation, immune dysfunction and coagulation disorders. The main inflammatory mediators that might contribute to the sepsis induced cardiac dysfunction are Tumor Necrosis Factor (TNF)-α, IL-1 and IL-6 [49]. In addition, PCT is positively correlated with both IL-6 and CRP, which are inflammatory indices [16], suggesting the inflammatory nature of PCT. PCT is also reported significantly elevated in AMI [50]. We hypotheses that elevated PCT either responses to sepsis induced by SARS-CoV-2 or acts as an inflammatory mediator involved in myocardial injury, triggering the caspases of signaling pathways which results in apoptosis/remodeling and release troponin (Figure 3). In our study, peak level of PCT were dramatically elevated, together with cTnI in non-survival patients, indicating PCT could be a key factor which causes myocardial injury.
Several studies have shown elevated D-Dimer is strongly associated with higher mortality in COVID-19 patients [51-53], which is supported by our study as well. D-Dimer is a degradation product of cross-linked fibrin clots lysis by plasmin. D-Dimer was first introduced into clinical practice for diagnosing Venous Thromboembolism (VTE) or disseminated intravascular coagulation. Later on, several clinical studies reported D-Dimer to be associated with cardiovascular diseases. Patients with coronary artery disease or with a history of myocardial infarction having high D-Dimer levels were found at high risk of cardiovascular death [54,55]. In the LIPID study (Long-Term Intervention with Pravastatin in Ischemic Disease), patients with myocardial infarction or unstable angina were followed up for 16 years, a high D-Dimer was found to be an independent predictor of cardiovascular disease mortality [56]. The endothelial cells play a key role in triggering the prothrombotic events. With aging, the imbalance between the generation of ROS and antioxidant systems causes endothelial dysfunction and venous thrombosis [57]. In our hypothesis model, it’s possible that the oxidative stress in COVID-19 patients triggers venous thrombosis and thereby D-Dimer increased (Figure 3). To further investigate the association between D-Dimer and myocardial injury, peak levels of both D-Dimer and cTnI were included. As shown in (Figure 2), both peak D-Dimer and cTnI were significantly elevated. Since the coagulation disorders is one of the mechanisms of sepsis induced cardiac dysfunction, based on our finding, D-Dimer could be an important factor for myocardial injury in COVID-19 patients.
Exaggerated systemic inflammation is correlated with lymphocytopenia in severe disease [58]. Lymphocytopenia is associated with a higher rate of non-survivors and critically ill patients with COVID-19, indicating a high vulnerability of lymphocytes by viral infection and destruction [59], which is actually critical in prevention of excessive inflammation after infection [60-62].
The occurrence of myocarditis in COVID-19 was reported due to systemic inflammation without evidence of direct cardiac viral infiltration [63]. Myocarditis is characterized by myocardial inflammatory infiltration and myocardial injury [64]. Virus has been reported as the most commonly cause of myocarditis [65,66]. The American Heart Association (AHA) recommends one or more cardiac imaging methods such as echocardiography or Cardiovascular Magnetic Resonance (CMR) as further testing for patients suspected with myocarditis [66]. Due to the difficulty of performing echocardiography with strict personal protective equipment and the potential risk to staff, the diagnosis of myocarditis in COVID-19 patients in our study may be not fully clear. The mechanism of viral myocarditis is hypothized as a combination of direct and inflammation-mediated cardiomyocytes injury [64]. The hypothized mechanism of inflammation-mediated cardiomyocytes injury in COVID-19 patients was presented in (Figure 3) in this study.
In conclusion, COVID-19 should be considered as an inflammatory disease. The pro-inflammatory cytokines or acute phase proteins induced by SARS-CoV-2 might initiate a cascade of signaling pathways which leads to cardiomyocyte apoptosis and troponin releasing, though SARS-CoV-2 could attack cardiomyocytes directly and result in cell death. There are several limitations in this study. This is a preliminary, one center assessment of clinical characteristics of myocardial injury in COVID-19 patients. Long-term follow-up, multi-center prospective and animal studies are necessary to verify our conclusion.
Grant support
R. John Solaro was supported in part by funding by the United States of America National Institutes of Health, National Heart, Lung and Blood Institute Grant PO1 HL 06246 and RO1 HL 119199.
Disclosure
R. John Solaro is a member to the Scientific Advisory Board of Cytokinetics, Inc. A Consultant to Pfizer, Inc. and Edgewise Therapeutics, Inc and a member of the Heart Failure Advisory Board of Amgen.
Authorship contribution statement
Rachel T. Scarl: Investigation, Methodology, Writing-original draft. Joan-Miquel Balada-LIasat: Data curation. Nicholas Nowacki: Investigation. R. John Solaro: Writing. JoAnna Williams: Investigation, Writing. Jieli Li: Investigation, Data curation, Formal analysis, writing.
References
- Solaro CR, Solaro RJ. Implications of the complex biology and micro-environment of cardiac sarcomeres in the use of high affinity troponin antibodies as serum biomarkers for cardiac disorders. J Mol Cell Cardiol. 2020; 145-158.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, et al. Fourth Universal Definition of Myocardial Infarction. Circulation. 2018; 138: e618-e651.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 1054-1062.
- Chen T, Wu D, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020; 368: m1091.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020; 1061-1069.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, et al. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020; 5: 811-818.
- Shi S, Qin M, Shen B, Cai Y, Liu T, et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA. Cardiol. 2020; 5: 802-810.
- Guan WJ, Ni ZY, Hu Y, Liang W, Ou C, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020.
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46: 846-848.
- Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020.
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341: 515-518.
- Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, et al. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive. Care. Med. 1998; 24: 185-188.
- Fang B, Meng QH. The laboratory’s role in combating COVID-19. Crit Rev Clin Lab Sci. 2020; 57: 400-414.
- Sturk A, Hack CE, Aarden LA, Brouwer M, Koster RR, et al. Interleukin-6 release and the acute-phase reaction in patients with acute myocardial infarction: a pilot study. J Lab Clin Med. 1992; 119: 574-579.
- Ostermann M, Ayis S, Tuddenham E, Lei K, Smith J, et al. Cardiac Troponin Release is Associated with Biomarkers of Inflammation and Ventricular Dilatation During Critical Illness. Shock. 2017; 47: 702-708.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323: 1239-1242.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020; 17: 259-260.
- Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Heart J. 1987; 113: 1333-1344.
- Katus HA, Remppis A, Looser S, Hallermeier K, Scheffold T, et al. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol. 1989; 21: 1349-1353.
- Rey JR, Valero SJ, Pinedo DP, Llorens JLM, Lopez-Sendon JL, et al. COVID-19 and simultaneous thrombosis of two coronary arteries. Rev Esp Cardiol (Engl Ed). 2020; 73: 676-677.
- Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020; 106: 1127-1131.
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, et al. Myocardial cell death in human diabetes. Circ Res. 2000; 87: 1123-1132.
- Al-Rasheed NM, Al-Rasheed NM, Hasan IH, Al-Amin MA, Al-Ajmi HN, et al. Simvastatin Ameliorates Diabetic Cardiomyopathy by Attenuating Oxidative Stress and Inflammation in Rats. Oxid Med Cell Longev. 2017; 2017: 1092015.
- Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010; 11: 31-39.
- Sukumaran V, Tsuchimochi H, Tatsumi E, Shirai M, Pearson JT. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1-7/Mas receptor cascade. Biochem Pharmacol. 2017; 144: 90-99.
- Althunibat OY, Al Hroob AM, Abukhalil MH, Germoush MO, Bin-Jumah M, et al. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019; 221: 83-92.
- Buturak A, Degirmencioglu A, Erturk M, Karakrut H, Demir AR, et al. Impact of increased admission lipid levels on periprocedural myocardial injury following an elective percutaneous coronary intervention. Coron Artery Dis. 2015; 26: 333-340.
- Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute Pneumonia and the Cardiovascular System. Lancet. 2013; 381: 496-505.
- Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, et al. Association between Influenza Vaccination and Cardiovascular Outcomes in High-Risk Patients: A Meta-Analysis. JAMA. 2013; 310: 1711-1720.
- Zhang X, Wu K, Wang D, Yue X, Song D, et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007; 365: 324-335.
- Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020; 53: 13-24.
- Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020; 130: 2202-2205.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020; 38: 1-9.
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46: 846-848.
- Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, et al. Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J Card Fail. 2020; 26: 470-475.
- Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007; 116: 1413-1423.
- Romero-Becerra R, Santamans AM, Folgueira C, Sabio G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int J Mol Sci. 2020; 21: 7412.
- Yoshida T, Das NA, Carpenter AJ, Izadpanah R, Kumar SA, et al. Minocycline reverses IL-17A/TRAF3IP2-mediated p38 MAPK/NF-κB/iNOS/NO-dependent cardiomyocyte contractile depression and death. Cell Signal. 2020; 73: 109690.
- Xu X, Han M, Li T, Sun W, Wang D, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020; 117: 10970-10975.
- Davila ML, Riviere I, Wang X, Bartido S, Park J, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014; 6: 224ra25.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395: 1033-1034.
- Zhazykbayeva S, Pabel S, Mugge A, Sossalla S, Hamdani N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys Rev. 2020; 12: 947-968.
- Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, et al. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem. 2004; 279: 2461-2469.
- Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, et al. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001; 276: 19715-19722.
- Lee YH, Lai CL, Hsieh SH, Shieh CC, Huang LM, et al. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res. 2013; 178: 411-422.
- Gjyshi O, Bottero V, Veettil MV, Dutta S, Singh VV, et al. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014; 10: e1004460.
- Vijayan AL, Vanimaya, Ravindran S, Saikant R, Lakshmi S, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017; 5: 51.
- Liu YC, Yu MM, Shou ST, Chai YF. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front Immunol. 2017; 8: 1021.
- Kafkas N, Venetsanou K, Patsilinakos S, Voudris V, Antonatos D, et al. Procalcitonin in acute myocardial infarction. Acute Card Care. 2008; 10: 30-36.
- Yao Y, Cao J, Wang Q, Shi Q, Liu K, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020; 8: 49.
- Gungor B, Atici A, Baycan OF. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am J Emerg Med. 2020.
- Zhang L, Yan X, Fan Q, Liu H, Liu X, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020; 18: 1324-1329.
- Morange PE, Bickel C, Nicaud V, Schnabel R, Rupprecht HJ, et al, AtheroGene Investigators. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol. 2006; 26: 2793-2799.
- Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, et al. Thrombogenic factors and recurrent coronary events. Circulation. 1999; 99: 2517-2522.
- Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, et al. D-Dimer Predicts Long-Term Cause-Specific Mortality, Cardiovascular Events, and Cancer in Patients with Stable Coronary Heart Disease: LIPID Study. Circulation. 2018; 138: 712-723.
- Wang Q, Zennadi R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int J Mol Sci. 2020; 21: 4259.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395: 1033-1034.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020; 71: 762-768.
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133: 775-787.
- Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002; 32: 1282-1291.
- Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016; 13: 3-10.
- Yao XH, Li TY, He ZC, Ping YF, Liu HW, et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020; 49: 411-417.
- Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008; 3: 127-155.
- Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013; 34: 2636-2648.
- Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, et al. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement from the American Heart Association. Circulation. 2020; 141: e69-e92.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.