Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Protective Effect of Propolis on Doxorubicin Induced Cardio and Nephrotoxicity
- Samaa Yassin Ali;
- Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Seuf University, Beni-Seuf, Egypt.
- Adel Abdel-moneim;
- Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Seuf University, Beni-Seuf, Egypt.
- Eman Salah Abdel-Reheim*
- Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Seuf University, Beni-Seuf, Egypt.

| Received | : | Aug 22, 2020 |
| Accepted | : | Sep 15, 2020 |
| Published Online | : | Sep 18, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Samaa Yassin A, Abdel-moneim A, Abdel-Reheim ES. Protective Effect of Propolis on Doxorubicin Induced Cardio- and Nephrotoxicity. Ann Cardiol Vasc Med. 2020: 3(1); 1028.
Keywords: Propolis; Doxorubicin; Cardiotoxicity; Nephrotoxicity; BNP; Troponin T.
Abstract
Doxorubicin, is an antineoplastic drug, induces objective tumor responses in solid tumors as well as in hematologic malignancies. Its major adverse effect are cardiotoxicity and nephrotoxicity. Bee products, such as honey, royal jelly, pollen, and propolis have great attention in recent years. Propolis contains a broad spectrum of compounds that have many biological activities. Cardiotoxicity as well as nephrotoxicity was induced in rats by intraperitoneal injection of doxorubicin (10 mg/kg) and protected or treated by (200 mg/kg/ day) of propolis extract administrated by gastric intubation for four weeks before or after doxorubicin administration. Cardiac biomarkers such as BNP, troponin T, LDH, CK, and AST in addition to creatinine and urea measurements, all ameliorated in the propolis; protected or treated groups. Cardiac and renal oxidative stress indicated by increased MDA and decreased antioxidant enzymes such as catalase, GSH and SOD in doxorubicin administrated group. This cardiac oxidation was improved in the other propolis pre- or post-treated groups. The measured lipid profile was also ameliorated with increased HDL in propolis groups. The study investigates that propolis has a protective and treatment effects against doxorubicin induced cardiotoxicity and nephrotoxicity mainly by its powerful antioxidant and lipid lowering efficacy.
Introduction
Doxorubicin (DOX) also known as Adriamycin it is an anthracyclin antineoplastic antibody was isolated in the early 1960s from the pigment-producing bacterium Streptomyces peucetius var. caesius [1]. Intercalation of DOX with a DNA molecule is recognized as the basic mechanism of its effect. Doxorubicin binds directly to DNA via intercalation between base pairs on the DNA helix. Doxorubicin also inhibits DNA repair by inhibiting topoisomerase II. These actions result in the blockade of DNA and RNA synthesis and fragmentation of DNA [2]. Doxorubicin is a powerful iron-chelator. The iron-doxorubicin complex can bind DNA and cell membranes producing free radicals that immediately cleave DNA and cell membranes. Although maximally cytotoxic in S phase, doxorubicin is not cell cycle-specific [3]. DOX was clinically used to treat a variety of cancers including breast, ovarian, and lymphoma [4]. Although doxorubicin remains one of the most important anticancer drugs in the clinic, unfortunately, its potent antitumor activity is also accompanied by sever tissue toxicities such as cardiotoxicity [4].
In 1991, long-term cardiotoxic effects were identified in patients with acute lymphoid leukemia in childhood [5]. Patients with childhood cancer and those treated with DOX have a high risk of developing symptomatic cardiac events at an early stage, and this risk remains high within 30 years after treatment. In addition, it is estimated that one in eight DOX-treated patients will be affected with severe cardiac disease [6].
Cardiotoxicity is defined as toxicity that affects the heart by the US National cancer institute. Although simple to understand in theory, this definition is very broad and in the case of cancer treatments, may encompass many effects, such as cardiac dysfunction and heart failure, myocardial ischemia or infarction, valvular abnormalities, pericardial disease, hypertension, and arrhythmias [7].
The exact mechanism of anthracyclin induced cardiotoxicity remains unclear, though it is likely to be multifactorial with different potential pathways involved that leads to cardiomyocyte death [6,8,9]. Until now, the main mechanisms that have been proposed by various research groups include oxidative stress, iron metabolism, Ca2+ hemostasis dysregulation, sarcomere structure alterations, gene expression modulation and apoptosis [8-11]. Since several mechanisms are involved in the development of cardiac toxicity, different strategies are being performed to prevent DOX-induced cardiomyopathy.
Renal dysfunction is among the noticeable negative effects of nephrotoxicity induced by doxorubicin increased permeability of the capillary of the glomerulus and tubular atrophy occur in the renal tissue of rats during DOX induction [12]. The toxicity of DOX on kidneys hasn’t been clarified yet it may be due to imbalanced oxidant-antioxidant systems, free radical formation, iron-dependent oxidative damage of biological macromolecules, membrane lipid peroxidation (LPO), and protein oxidation. The disturbance in oxidant-antioxidant systems, which has been demonstrated with LPO and protein oxidation, results in tissue injury [13].
Propolis or bee glue, is a dark-colored resinous substance collected by honeybees from leaf buds and cracks in the bark of various tree species [14]. Propolis has been used as a folk medicine since 300 BC [15]. Recently, numerous biological properties of propolis have been reported including antibacterial, antifungal, antiviral, immunoregulatory, anti-oxidative, antitumor, hepatoprotective and anti-inflammatory [14]. Because of the wide range of biological activities, propolis has recently been extensively used in food and beverages to improve health and prevent diseases [15]. The chemical composition of propolis is quite complicated and over 300 components have been identified including flavonoids, phenolic acids, esters, terpenoids, steroids and amino acids [16]. The data administrated in this search study the protective or treatment effect of propolis against cardioto-and nephrotoxicity induced by doxorubicin.
Materials and methods
Animals
A total of 32 male albino Wistar rats (Rattus norvegicus) weighing (110-130 g) were obtained from the Animal Farm of the Egyptian Holding Company for Biological Products and Vaccines, Cairo, Egypt. Animals were housed in plastic cages and maintained under standard conditions of temperature, humidity and 12 h light/dark cycle along the experimental period. Rats were provided with a pellet of concentrated diet containing all the necessary nutritive elements. Rats were left to acclimatize for 1 week before starting the experiment. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), Beni-Suef University, Egypt (BSU/FS/2015/11).
Chemicals
Doxorubicin hydrochlorid (10 mg vial for injection) is the active ingredient of adriablastina. It is produced from Pharmacia Company and purchased from local pharmacy.
Preparation of extract
Crude propolis was obtained from faculty of agriculture, Fayoum University and was prepared according to the method of Mani [17].
Experimental designs
The rats were divided equally into 4 groups (each group contains 8 rats) as following:
Group I (normal control): rats were kept under the standard condition for four weeks then scarified at the end of the experiment.
Group II (doxorubicin): It is the positive control group; rats were injected once with intraperitoneal doxorubicin (10 mg /kg) [4] then sacrificed after 48 hours.
Group III (propolis+ doxorubicin): It is the protected group, rats were received propolis (200 mg /kg) [14] daily for four weeks by gastric intubation then injected with intraperitoneal doxorubicin (10 mg/kg) and sacrificed after 48 hours.
Group IV (doxorubicin+ propolis): It is the treated group, rats were injected with intraperitoneal doxorubicin (10 mg /kg) then after 2 days, started to receive propolis (200 mg / kg) daily for four weeks and sacrificed at the end.
At the end of experiment, overnight fasted rats were sacrificed under light diethyl ether anesthesia. The blood samples were collected and serum was obtained after centrifugation of blood samples at 3000 rpm for 10 minutes. Heart and kidney were directly separated after scarification, washed in ice-cold saline then was homogenized in saline (10% W/V) and the homogenates were stored at -30°C until oxidation parameters were assayed.
Biochemical measurements
Creatinine kinase (CK) and lactate dehydrogenase (LDH) were determined kinetically using reagent kits purchased from Spectrum Company (Egypt). Glutamic oxaloacetate transaminase (GOT) and Creatinine concentration were determined kinetically using reagent kits purchased from Bioscope Diagnostics Chemical Company (Egypt). Troponin T was determined using an enzyme-linked immunosorbent assay (ELISA) by the method of Bhaskar and Rao [18]. Brain natriuretic peptide (BNP) was determined by the method of Bibbins-Domingo et al. [19]. Urea concentration was determined enzymatically colorimetric using kits purchased from Diamond Diagnostics Chemical Company (Germany). Cholesterol, triglycerides and high density lipoprotein (HDL) cholesterol were measured by colorimetric reagent kits purchased from Spinreact Company (Spain). Low density lipoprotein (LDL) and very low density lipoprotein (vLDL) cholesterol concentration were determined according to Friendewald et al. and Norbert reactions respectively:
LDL-cholesterol= Total cholesterol – Triglycerides / 5 – HDL-cholesterol
vLDL-cholesterol= Triglycerides / 5
Heart and kidney homogenates were used to assay oxidation state of the organs. Malondialdehyde (MDA) (lipid peroxidation marker) was determined by the method of Preuss et al. Catalase (CAT) and superoxide dismutase (SOD) activities were determined by the methods of Cohen et al. and Kakkar et al. respectively. Glutathione (GT) content was determined by the method of Beutler et al.
Statistical analysis
The data were analyzed using oneway anova followed by Tukey-Kramer method for post-hoc analysis to compare various groups with each other. The results were expressed as mean ± standard error (SE). Statistical significance interval is considered as P < 0.05 for all data. All results were analyzed using Statistical Package for Social Science (SPSS) version 20 software.
Results
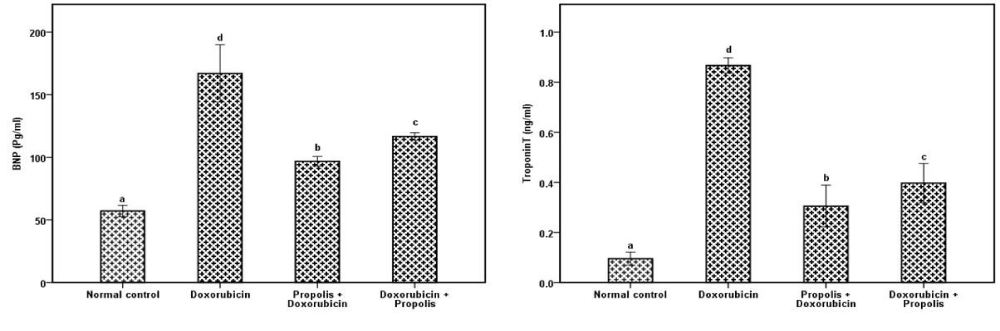
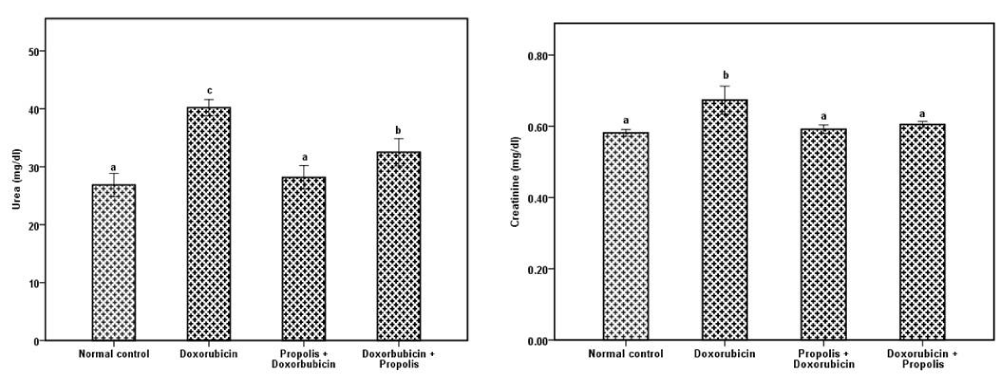
Administration of doxorubicin induced cardiac toxicity. The cardiac biomarkers such as CK, LDH, GOT were significantly elevated in doxorubicin group in comparison with normal group while in groups administrated propolis before or after doxorubicin administration showed a significant decrease (P<0.001) compared to doxorubicin group (Table 1). Troponin T, BNP were significantly (P<0.001) elevated in doxorubicin group in comparison with normal group while in groups administrated propolis before or after doxorubicin administration show significant decrease (P<0.001) compared to doxorubicin group (Figure 1). Table 2 showed that propolis ameliorate the levels of lipid profile in the groups of rats administrated it before or after doxorubicin. Table 3 showed that doxorubicin administration produced oxidative stress in cardiac tissue as indicated by increased MDA (P<0.001) while cardiac CAT, SOD and GSH were decreased significantly in comparison with normal control rats. These were ameliorated totally in propolis protected or treated groups. Kidney function measured by creatinine and urea was designated in Figure 2 while table 4 indicated the toxic effect of doxorubicin in the renal oxidation. This was presented by significant elevated MDA and decreased antioxidant enzyme activities, improved by propolis. On the other hand, GSH was not significantly changed in the all groups.
Discussion
Doxorubicin is a DNA topoisomerase II (Top2) targeted therapy [20]. The antitumor activity of doxorubicin is mediated by poisoning of Top2 (primarily the Top2α isozyme) and formation of Top2α-DNA covalent adducts. Doxorubicin mediated redox cycling and therefore ROS generation [21]. Unbalanced production and degradation of ROS and RNS can result in accumulation of these reactive species, commonly referred to as oxidative stress. ROS are usually detoxified by intracellular enzymes, such as glutathione reductase, superoxide dismutase, and catalase [22]. Exposure of macromolecules (lipid, proteins, DNA, etc.) to reactive species results in oxidative modifications with deleterious effects [23,24]. Administration of doxorubicin decrease antioxidant enzymes activity [25] and decreased levels of cardiac glutathione or superoxide dismutase and catalase activities. The myocardium injury evidenced by lipid peroxidation occurs as a result of the increase of the reactive oxygen species (ROS) production, including superoxide (O2-) and hydroxyl radicals (OH˙) as well as other non-radicals such as hydrogen peroxide (H2O2), singlet oxygen (O2), etc. [1,6,11]. Excessive production of free radicals damages the myocardium which leads to increased membrane permeability and leads to the increase of LDH, CK and AST concentration in serum [26].
The mechanism of doxorubicin induced cardiotoxicity include oxidative stress, iron metabolism, Ca2+ homeostasis dysregulation, sarcomere structure alterations, gene expression modulation, and apoptosis [9,10]. Doxorubicin induced diastolic dysfunction related to other pathologies. Increased oxidative stress and intracellular Ca2+ dysregulation is commonly present and closely interconnected [8,11].
Troponins are myocardial regulatory proteins, which regulate the calcium mediated actin and myosin interaction. Troponin-T is widely used as specific marker to diagnose myocardial infarction [27]. In this study propolis minimized the elevated effect of doxorubicin on troponin T when it administrated before or after doxorubicin. The release of BNP reflects the alterations in left ventricular potency as a response to B-adrenergic stimulation on the heart. These effects are mediated through B-1 and B-2 adrenoceptors, mediating the positive inotropic and chronotropic effects of B adrenoceptor agonists [28]. BNP has been used not only as a biomarker for the diagnosis of heart failure, but also to detect asymptomatic left ventricle dysfunction and to predict the prognosis [29-31]. In this study administration of doxorubicin increase BNP levels as was previously reported by Cappetta et al. [32].
Propolis is a powerful antioxidant rich with flavonoids capable of scavenging free radicals and thereby protecting the cell membrane against lipid peroxidation [11]. The composition of propolis is variable; however, one of its major components is caffeic acid phenethyl ester (CAPE) which is able to block ROS production in several systems [10]. It is also probable that propolis restores antioxidant enzyme function via upregulation of the activity or expression of Nrf2, an important intracellular transcription factor, released from its repressor (Keap1) under oxidative or xenobiotic stress [33]. The released Nrf2 binds to the antioxidant response element (ARE) in the promoter region of cytoprotective genes and induces their expression. The transcribed genes subsequently induce the expression of free radical-scavenging enzymes to neutralize, detoxify, and eliminate the cytotoxic oxidants [34].
Fuliang study showed that propolis extracts lead to decrease the level of lipid peroxidation (MDA), total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL), very low-density lipoprotein cholesterol (vLDL) and increased serum levels of high density lipoprotein (HDL) and superoxide dismutase (SOD). This suggests that propolis can modulate the metabolism of blood lipid, leading to decreased outputs of lipid peroxidation and scavenge the free radicals. That is agreed with our results, as administration of doxorubicin increase lipid peroxidation while administration of propolis before or after doxorubicin administration enhance lipid metabolism by lowering cholesterol, triglycerides, vLDL and LDL with increasing HDL concentration. This may be explained by the upregulation of ABCA 1 gene expression associated with increased HDL-C levels and restoration of lipid profiles in animals Also, the protective effect of propolis may be due to its phenolic components preserving the myocardium structural and functional integrity of the contractile apparatus and thus preventing oxidative injury of cardiac muscles.
In a study for Köse et al. [13] nephrotoxicity was shown in doxorubicin administered rats by producing renal oxidative stress and excessive release of free radicals as presented in this study. Baykara et al. [35] evaluate nephroprotective effect of propolis by decreased serum creatinine, urea and improving renal oxidation, that is agreed with our study. In a study by Promsan et al. [36] investigate that pretreatment with pinocembrin (5,7-dihydroxyfl avone) as one of the main fl avonoids of propolis [37] improved renal function and reduced oxidative stress and apoptotic conditions These findings indicate that pinocembrin has a protective effect against nephrotoxicity which may be due in part to its antioxidant and anti-apoptotic effects. It attenuates the increase in oxidative stress and modulates the antioxidant enzymes via the Nrf2/HO-1, NQO1 pathways, thereby leading to reduce protein-related apoptosis results in improved renal function.
In Garoui et al. [38] study, they predicted that administration of propolis to the diet of cobalt treated rats ameliorates the kidney impairment induced by cobalt as suggested by a significant restoration of plasma urea, creatinine levels and the creatinine clearance. This might be due to the accelerated regeneration of parenchymal cells under the influence of various bioactive compounds like flavonoids and esters present in propolis that helped to prevent membrane fragility and subsequently decreased the leakage of marker enzymes into circulation and this explain the significant decrease in creatinine and urea levels in the groups treated with propolis.
The significant increase in BNP level in doxorubicin group may be related to nephrotoxic as well as cardiotoxic effects of doxorubicin. Renal failure may result in increased concentrations of BNP as the cardiac ventricles rapidly release BNP paired with NT-proBNP to increase vasodilation and renal output of sodium and water to counter the increased fluid volume resulting from decreased renal function [39].
Conclusion
At the end of the study we investigate that propolis is a powerful antioxidant, lipid lowering agent with membrane stabilizing effect that explain cardio-and nephron-protective and treatment effects.
References
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Progress in Cardiovascular Diseases. 2007; 49: 330-352.
- Gabizon AA, Patil Y, La-Baeck NM. New insights and evolving role of pegylated liposomal doxorubicin cancer therapy. Drug resistance updates, 2016; 29: 90-106.
- Dorr RT, Von-Hoff DD. Drug monographs. Cancer chemotherapy handbook. 2nd ed. Norwalk, Conneticut: Appleton and Lange. 1994; 395-416.
- Alrushaid S, Sayre CL, Yanez JA, Forrest ML, Senadheera SN, et al. Pharmacokinetic and Toxicodynamic Characterization of a Novel Doxorubicin Derivative. Pharmaceutics. 2017; 13; 9.
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med, 1991; 324: 808.
- Raj S, Franco SI, Lipshult SE. Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Current Treatment Options in Cardiovascular Medicine. 2014; 16: 315.
- Sadurska E. Current views on anthracycline cardiotoxicity in childhood cancer survivors. Pediatric Cardiology. 2015; 36: 1112-1119.
- Salazar-Mendiguchia J, Gonzalez-Costello J, Roca J, ArizaSole A, Manito N, et al. Anthracycline-mediated cardiomyopathy: basic molecular knowledge for the cardiologist. Arch Cardiol Mex. 2014; 84: 218-23.
- Ghigo A, Li M, Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochimica et Biophysica Acta. 2016; 1863 (7 Pt B): 1916-1925.
- Geiger S, Lange V, Suhl P, Heinemann V, Stemmler HJ. Anticancer therapy induced cardiotoxicity: Review of thl literature. Anti-Cancer Drugs, 2010; 21: 578-590.
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol, 2012; 52: 1213-1225.
- Molehin OR, Adeyanju AA, Adefegha SA, Oyeyemi AO, Idowu KA. Protective mechanisms of protocatechuic acid against doxorubicin-induced nephrotoxicity in rat model: Journal of basic and clinical physiology and pharmacology. 2019; 30.
- Köse E, Oğuz F, Vardi N, Sarihan ME, Beytur A, et al. Therapeutic and protective effects of montelukast against doxorubicininduced acute kidney damage in rats. Iran J Basic Med Sci. 2019; 22: 407-411.
- Sameni HR, Ramhormozi P, Bandegi AR, Taherian AA, Majid M, et al. Effects of ethanol extracts of propolis on histopathological changes and antioxidant defense of kidney in a rat model for type 1 diabetes mellitus. J Diabetes Investig. 2016.
- Toreti VC, Sato HH, Pastore GM, Park YK . Recent prog-ress of propolis for its biological and chemical compositionsand its botanical origin.Evid Based Complement AlternatMed, 2013: 1-13.
- Tolba MF, Azab SS, Khalifa AE. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotcvtive and cardioprotective. IUBMB Life. 2013; 65: 699-709.
- Mani F, Damasceno HCR, Novelli ELB, Martins EAM, Sforcin J M. Propolis: Effect of different concentrations, extracts and intake period on seric biochemical variables. Journal of Ethnopharmacology, 2006; 105: 95-98.
- Bhaskar I, Rao SB. New, simple and cheap alternative to troponin test for diagnosis of acute myocardial infarction. Indian J exp boil. 2002; 40: 628-630.
- Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. “Adolescent overweight and future adult coronary heart disease.” N Engl J Med. 2007; 357: 2371-2379
- Azarova AM, Lyu YL, Lin CP, Tsai YC, Lau JY, et al. Roles of DNA topoisomerase II isozymes inchemotherapy andsecondary malignancies. Proc. Natl. Acad. Sci. USA. 2007; 104: 11014-11019.
- Lyu YL, Liu LF. Doxorubicin cardiotoxicity revisited: ROS versus Top2. In: Liu XY, Pestka S, Shi YF, editors. Recent advances in cancer research and therapy. Oxford: Elsevier. 2012: 351-369.
- Van Raamsdonk J M, Hekimi S. Reactive oxygen species and aging in Caenorhabditis elegans: causal or causal relationship? Antioxidants and Redox Signaling, 2010; 13: 1911-1953.
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. oxidative DNA damage: mechanisms, mutation, and disease. FASEB Journal. 2003; 17: 1195-1214
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radical Biology and Medicine. 2007; 42: 153-164.
- Afsar T, Razak S, Batoo KM, Khan MR. Acacia hydaspica R. Parker prevents doxorubicin-induced cardiac injury by attenuation of oxidative stress and structural Cardiomyocyte alterations in rats MC Complementary and Alternative Medicine. 2017; 17: 554.
- Swamy AV, Wangikar U, Koti BC, Tippeswamy AHM, Ronad PM, et al. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian Journal of Pharmacology. 2011; 43: 507-511.
- Sharma S, Jackson PG, Makan J. Cardiac troponins. J Clin Pathol. 2004; 57: 1025-1026
- Stuveling EM, Hillege HL, Bakker S J, Gans RO, De Jong PE, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003; 63: 654-661.
- Minotti G. Pharmacology at work for cardio-oncology: ranolazine to treat early cardiotoxicity induced by antitumor drugs. J pharmacol Exp Ther. 2013; 346: 343-349.
- Ishigaki T, Yoshida T, Izumi H, Fujisawa Y, Shimizu S, et al. Different implication of elevated B-type natriuretic peptide level in patients with heart failure with preserved ejection fraction and in those with reduced ejection fraction. Echocardiography. 2015; 32: 623-629
- Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016; 118: 1008-1020
- Cappetta D, Esposito G, Coppini R, Piegari E, Russo R, et al. Effects of ranolazine in a model of doxorubicin-induced left ventricle diastolic dysfunction. Br J Pharmacol. 2017; 174: 3696-3712.
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, et al. The antioxidant defense system Keap1–Nrf2 comprises a multiple sensing mechanism for responding toa wide range of chemical compounds. Mol. Cell. Biol. 2009; 29: 493-502.
- Erejuwa OO, Sulaiman SA, Ab Wahab MS. Honey: a novel antioxidant. Molecules. 2012; 17: 4400-4423.
- Baykara M, Silici S, Özçelik M, Güler O, Erdoğan N, Bilgen M. In vivo nephroprotective efficacy of propolis against contrast-induced nephropathy. Diagnostic and Interventional Radiology. 2015; 21: 317.
- Promsan S, Jaikumkao K, Pongchaidecha A, Chattipakorn N, Chatsudthipong V, Arjinajarn P, Lungkaphin A. Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Canadian journal of physiology and pharmacology. 2016; 94: 808-818.
- Cauich-Kumul R, Campos MRS. Bee Propolis: Properties, Chemical Composition, Applications, and Potential Health Effects. In Bioactive Compounds. Mexico: Woodhead Publishing. 2019: 227-243.
- Garoui EM, Troudi A, Fetoui H, Soudani N, Boudawara T, et al. Propolis attenuates cobalt induced-nephrotoxicity in adult rats and their progeny. Experimental and toxicologic pathology. 2012; 64: 837-846.
- Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. American journal of clinical pathology. 2010; 133: 14-23.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.