Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case Report
- |
- Open Access
- |
- ISSN: 2639-4383
Severe Pulmonary Artery Hypertension after Displacement of Endovascular Stent because of Budd-Chiari Syndrome
- Xuefeng Lin;
- Department of Cardiovascular Surgery, Nanfang Hospital, Southern Medical University, 510000 Guangzhou, China.
- Peng Zhu;
- Department of Cardiovascular Surgery, Nanfang Hospital, Southern Medical University, 510000 Guangzhou, China.
- Shaoyi Zheng*
- Department of Cardiovascular Surgery, Nanfang Hospital, Southern Medical University, 510000 Guangzhou, China.

| Received | : | Feb 08, 2021 |
| Accepted | : | Mar 08, 2021 |
| Published Online | : | Mar 11, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Lin X, Zhu P, Zheng S. Severe Pulmonary Artery Hypertension after Displacement of Endovascular Stent because of Budd-Chiari Syndrome. Ann Cardiol Vasc Med. 2021: 4(1); 1046.
Keywords: Inferior Vena Cava; Syndrome; Hypertension; Syndrome; Stent, Acute; Chronic.
Abstract
We reported a case of a patient who has repeated exercise dyspnea for 1 year and deteriorated for 4 mouths after successful percutaneous angioplasty and the implantation of a stent in the Inferior Vena Cava (IVC) because of Budd-Chiari Syndrome (BCS). Stent displacement which resulted in Pulmonary Artery Hypertension (PAH) was observed.
Case report
Budd-Chiari Syndrome (BCS) comprises a heterogeneous group of conditions characterized by partial or complete hepatic venous outflow obstruction [1]. Its clinical manifestations can be classified as fulminant, acute, subacute, chronic [2]. In recent years, endovascular interventional therapy technology has developed rapidly, Percutaneous Transluminal Angioplasty (PTA) and Endovascular Stent Implantation (ESI) and Trans Jugular Intrahepatic Portosystemic Shunt (TIPS) gradually developed into the first choice for BCS. This case report was evaluated by our Institutional Review Board (IRB) and determined that it did not require IRB review and informed consent was waived [3].
A 50-year-old man with a 4-month history of aggravating exercise dyspnea and shortness of breath was admitted to our hospital. His symptom had developed during 1 year before admission. There was no history of alcohol or smoke use. Percutaneous angioplasty and implantation of a bare metal stent in Inferior Vena Cava (IVC) was successfully operated 6 years ago. This patient received regularly postoperative follow-up in our hospital since 2016, rivashaban has been taken for anticoagulant therapy in postoperative period. Since a mass had been reported in March 2020, Warfarin has been taken for anticoagulant.
On physical examination, slightly cyanotic was observed in lips. The abdomen was soft, liver could not be touched, with hypoactive bowel sounds. There was no peripheral edema or jugular venous distention, and heart sounds were normal.
Initial laboratory examinations revealed hematocrit of 58.8%, hemoglobin of 198 g/L, a platelet count of 105*10^9/L, a white blood cell count of 5.7*10^9/L, Serum Alanine Transaminase (ALT) at 28 U/L, Aspartate Aminotransferase (AST) at 29 U/L, total bilirubin at 44.8 μmol/L, direct bilirubin at 13.3 μmol/L, albumin at 37.2g/L, and an international normalized ratio of 2.54. D-dimer at 0.32 mg/L FEU. Arterial blood gas analysis showed hypoxemia, its partial pressure of oxygen was 56 mmHg.
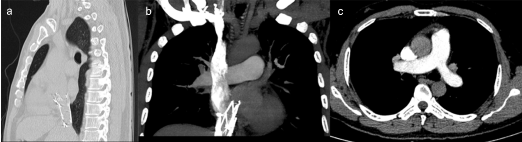
Computed Tomographic Angiography (CTA) scan demonstrated hyper dense stent shadow in the lumen of IVC, the proximal end configuration about 44 mm above diaphragm, without any evidence of obviously pulmonary embolism (Figure 1).
Transthoracic echocardiography revealed an abnormal echo mass (20*13 mm) originating from the orifice of the IVC to the septal tricuspid valve, causing mild tricuspid valve insufficiency. Importantly, severe pulmonary artery hypertension was observed for the first time.
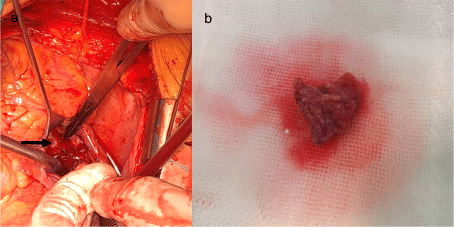
The right atrial mass was removed under extracorporeal circulation but without clamping the aorta. After midline sternotomy, direct mean pulmonary artery pressure (mPAP) was measured approximately 70mmHg, bicaval and ascending aortic cannulation were accomplished, and total cardiopulmonary bypass was initiated. Right atriotomy was made, and an endovascular stent was suspended in the right atrium, originating from the orifice of the IVC, the FreeFlo proximal end configuration was oppressing the anterior and septal tricuspid valve which caused tricuspid valve insufficiency, another end configuration was rubbing the free wall of the right atrial near to septal tricuspid valve, bringing out a loose and actinian mass adhere to the wall (Figure 2). Histology examination revealed the mass was a thrombus. A natural function of tricuspid valve was detected after removing the stent exposed in the right atrium. Another mPAP still about 70mmHg was measured after terminating cardiopulmonary bypass. Macitentan has been taken in postoperative which maintained the pulmonary pressure about 50-70mmHg. Moreover, the duration of ventilation was about 28 hours, and the length of stay in Intensive Care Unit was about 3 days. The postoperative course was uneventful and repeat transthoracic echocardiography demonstrated no abnormal findings.
Figure 1: (a) Stent displaced into right atrium in median sagittal section.
(b) The proximal end configuration about 44 mm above diaphragm in coronal section.
(c) Computed tomographic angiography cannot find evidence of pulmonary embolism in main pulmonary artery.
Figure 2: (a) The FreeFlo proximal end configuration was oppressing the anterior and septal tricuspid valve, arrow was pointing out the proximal end configuration.
(b) Loose thrombus removed from right atrium.
Comment
Budd–Chiari Syndrome (BCS) is a rare disease characterized by hepatic venous outflow tract obstruction3. The obstruction of the hepatic venous outflow tract results in increased hepatic sinusoidal pressure and portal hypertension. Most cases of BCS are caused either by hepatic vein thrombosis or mechanical outflow obstruction [4]. The major treatment options include anticoagulation, thrombolysis, percutaneous recanalization, Transjugular Intrahepatic Portosystemic Shunt (TIPS), surgery and liver transplantation. In recent years, endovascular interventional therapy has became the first therapeutic choice for BCS due to its minimal invasiveness.
Pulmonary artery hypertension (PAH), defined as elevated pulmonary artery pressure, is common in the general population and associated with increased mortality. According to the ESC Guidelines on pulmonary hypertension, pulmonary hypertension owing to chronic thrombotic and/or embolic disease has been classified in an independent group, which was introduced as Chronic Thromboembolic Pulmonary Hypertension (CTEPH) [5,6]. CTEPH results from the obstruction of the pulmonary vascular bed by nonresolving thromboembolic.
In this case, there was a chronic abrasion between stent and wall of the right atrial due to the displacement of stent, resulting in the loose mass comprised of thrombus, which was happening when the International Normalized Ratio (INR) was 1.0-1.5. However, along with the heartbeat, newly forming thrombus has shed and subsequently flowed to distal pulmonary artery. Undoubtedly, the strength of anticoagulation therapy was unsatisfactory. However, what the appropriate range of INR the clinicians should maintain still remained poorly understood. Distal obstructions led to the development of vascular remodeling and elevated pulmonary pressure. On the other hand, distal obstructions also led to chronic hypoxia, thereby increasing a great number of hemoglobin. Therefore we suspected that it was an obsolete embolism so that level of D-dimer was negative on admission. It was fortunate that tricuspid valve insufficiency was revealed because of the displacement of stent, otherwise severe pulmonary hypertension from this patient couldn’t be demonstrated or might be find out in a very end stage of this disease. We only snipped out the stent exposed in right atrium, and the propose of our procedure was to avoid new embolism. Nowadays, endovascular interventional treatment is introduced as an advanced therapy for BCS, which needs regular follow-up. Unfortunately, this patient suffered from stent displacement-induced endothelium injury, chronic abrasion was one of the most important factors that led to thrombogenesis. Consequently, it is highly recommended for BCS patients receiving stent implantation to have regular long-term follow-up, which should not only focus on the routine auxiliary examination, but also the position of stent. Moreover, anticoagulant played an important role in cases that endothelium injury probably occurred because of stent displacement, it would prevent from pulmonary hypertension which was caused by thromboembolic in distal pulmonary artery. As for postoperative treatment strategy, we not only anticoagulated by Warfarin but also Macitentan used for the treatment of pulmonary arterial hypertension.
Above all, timely surgical intervention will be a promising strategy once postoperative complications appear in circulation system such as pulmonary hypertension, valve insufficiency, embolic event, bleeding and so on. In addition, it also needs multidisciplinary discussion with cardiologist, interventionist, respiratory physician, radiologist, ultrasound physician.
In conclusion, we reported a case of pulmonary artery hypertension due to the displacement of endovascular stent, which was a potentially complication caused by BCS treatment. Thus, if pulmonary artery hypertension or exercise dyspnea and shortness of breath has no recognizable cause after IVC stent implantation, chronic thromboembolic pulmonary hypertension should be considered.
References
- Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: A review by an expert panel. J Hepatol. 2003; 38: 364-371.
- Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd-Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005; 2: 182-190.
- Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018; 12: 168-180.
- Okuda K. Inferior vena cava thrombosis at its hepatic portion (obliterative hepatocavopathy). Semin liver dis. 2002; 22: 15-26.
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54: S43-S54.
- Albani S, Biondi F, Stolfo D, Lo GF, Sinagra G. Chronic Thromboembolic Pulmonary Hypertension (CTEPH): What do we know about it?. A comprehensive review of the literature. J Cardiovasc Med (Hagerstown). 2019; 20: 159-168.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.